Protein catabolism on:
[Wikipedia]
[Google]
[Amazon]
In
molecular biology
Molecular biology is a branch of biology that seeks to understand the molecule, molecular basis of biological activity in and between Cell (biology), cells, including biomolecule, biomolecular synthesis, modification, mechanisms, and interactio ...
, protein catabolism is the breakdown of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s into smaller peptides and ultimately into amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s. Protein catabolism is a key function of digestion
Digestion is the breakdown of large insoluble food compounds into small water-soluble components so that they can be absorbed into the blood plasma. In certain organisms, these smaller substances are absorbed through the small intestine into th ...
process. Protein catabolism often begins with pepsin
Pepsin is an endopeptidase that breaks down proteins into smaller peptides and amino acids. It is one of the main digestive enzymes in the digestive systems of humans and many other animals, where it helps digest the proteins in food. Pe ...
, which converts proteins into polypeptides. These polypeptides are then further degraded. In humans, the pancreatic proteases include trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the dig ...
, chymotrypsin
Chymotrypsin (, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenu ...
, and other enzymes. In the intestine, the small peptides are broken down into amino acids that can be absorbed into the bloodstream. These absorbed amino acids can then undergo amino acid catabolism, where they are utilized as an energy source or as precursors to new proteins.
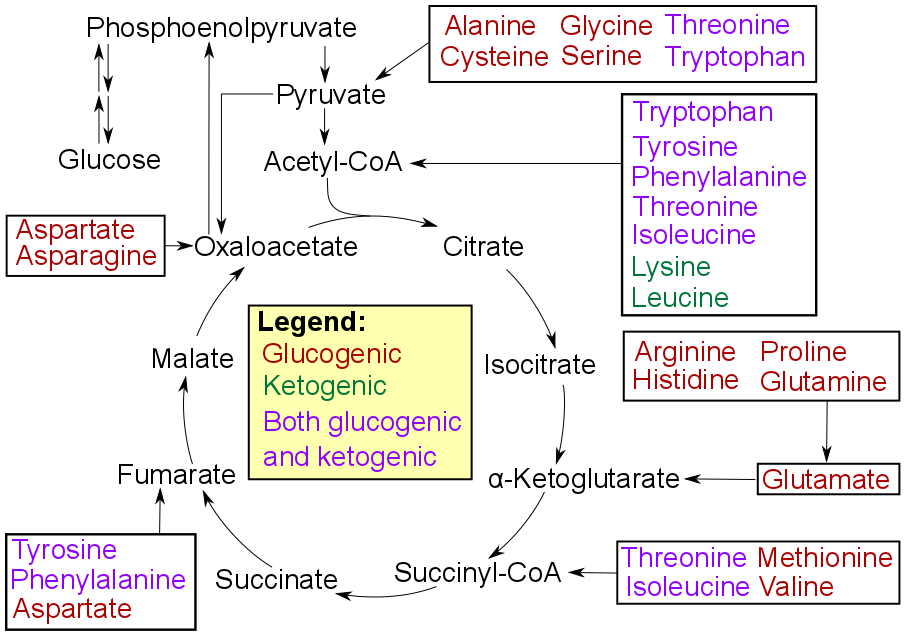
The amino acids produced by catabolism may be directly recycled to form new proteins, converted into different amino acids, or can undergo amino acid catabolism to be converted to other compounds via the Krebs cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions that release the energy stored in nutrients through acetyl-CoA oxidation. The e ...
.
Interface with other metabolic and salvage pathways
Protein catabolism produces amino acids that are used to form other proteins or oxidized to meet the energy needs of the cell. The amino acids that are produced by protein catabolism can then be further catabolized in amino acid catabolism. Among the several degradative processes for amino acids areDeamination
Deamination is the removal of an amino group from a molecule. Enzymes that catalysis, catalyse this reaction are called deaminases.
In the human body, deamination takes place primarily in the liver; however, it can also occur in the kidney. In s ...
(removal of an amino group), transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids.This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential a ...
(transfer of amino group), decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
(removal of carboxyl group), and dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At ...
(removal of hydrogen). Degradation of amino acids can function as part of a salvage pathway, whereby parts of degraded amino acids are used to create new amino acids, or as part of a metabolic pathway whereby the amino acid is broken down to release or recapture chemical energy. For example, the chemical energy that is released by oxidization in a dehydrogenation reaction can be used to reduce NAD+ to NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an ade ...
, which can then be fed directly into the Krebs/Citric Acid (TCA) Cycle.
Protein degradation
Protein degradation differs from protein catabolism. Proteins are produced and destroyed routinely as part of the normal operations of the cell.Transcription factor
In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription (genetics), transcription of genetics, genetic information from DNA to messenger RNA, by binding t ...
s, proteins that help regulate protein synthesis, are targets of such degradations. Their degradation is not a significant contributor to the energy needs of the cell. The addition of ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 19 ...
(ubiquitylation) marks a protein for degradation via the proteasome
Proteasomes are essential protein complexes responsible for the degradation of proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases. Proteasomes are found inside all e ...
.

Amino acid degradation
Oxidative deamination is the first step to breaking down the amino acids so that they can be converted to sugars. The process begins by removing the amino group of the amino acids. The amino group becomesammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
as it is lost and later undergoes the urea cycle
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic.
The urea cycle converts highl ...
to become urea, in the liver. It is then released into the blood stream, where it is transferred to the kidneys, which will secrete the urea as urine. The remaining portion of the amino acid becomes oxidized, resulting in an α-keto acid
In organic chemistry, keto acids or ketoacids (also called oxo acids or oxoacids) are organic compounds that contain a carboxylic acid group () and a ketone group ().Franz Dietrich Klingler, Wolfgang Ebertz "Oxocarboxylic Acids" in Ullmann's En ...
. The alpha-keto acid will then proceed into the TCA cycle, in order to produce energy. The acid can also enter glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form ...
, where it will be eventually converted into pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic ...
. The pyruvate is then converted into acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidation, o ...
so that it can enter the TCA cycle and convert the original pyruvate molecules into ATP, or usable energy for the organism.
Transamination leads to the same result as deamination: the remaining acid will undergo either glycolysis or the TCA cycle to produce energy that the organism's body will use for various purposes. This process transfers the amino group instead of losing the amino group to be converted into ammonium. The amino group is transferred to α-ketoglutarate, so that it can be converted to glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that ...
. Then glutamate transfers the amino group to oxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes ...
. This transfer is so that the oxaloacetate can be converted to aspartate or other amino acids. Eventually, this product will also proceed into oxidative deamination to once again produce alpha-ketoglutarate, an alpha-keto acid that will undergo the TCA cycle, and ammonium, which will eventually undergo the urea cycle.
Transaminase
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.
Function and mechanism
An amino acid con ...
s are enzymes that help catalyze the reactions that take place in transamination. They help catalyze the reaction at the point when the amino group is transferred from the original amino acid, like glutamate to α-ketoglutarate, and hold onto it to transfer it to another α-ketoacid.
Factors determining protein half-life
Some key factors that determine overall rate include protein half-life, pH, and temperature. Protein half-life helps determine the overall rate as this designates the first step in protein catabolism. Depending on whether this step is short or long will influence the rest of the metabolic process. One key component in determining the protein half-life is based on theN-end rule The ''N''-end rule is a rule that governs the rate of proteolysis, protein degradation through recognition of the N-terminal residue of proteins. The rule states that the N-terminus, ''N''-terminal amino acid of a protein determines its half-life (t ...
. This states that the amino acid present at the N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
of a protein helps determine the protein's half-life.
Further reading
*See also
*Amino acid synthesis
Amino acid biosynthesis is the set of biochemical processes (metabolic pathways) by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to ...
*Anabolism
Anabolism () is the set of metabolic pathways that construct macromolecules like DNA or RNA from smaller units. These reactions require energy, known also as an Endergonic reaction, endergonic process. Anabolism is the building-up aspect of metabo ...
*Metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
*Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Protein degradation is a major regulatory mechanism of gene expression and contributes substantially to shaping mammalian proteomes. Uncatalysed, the hydrolysis o ...
References
{{DEFAULTSORT:Protein Catabolism Metabolism Proteins as nutrients