Potential temperature on:
[Wikipedia]
[Google]
[Amazon]
The potential temperature of a parcel of fluid at pressure is the temperature that the parcel would attain if
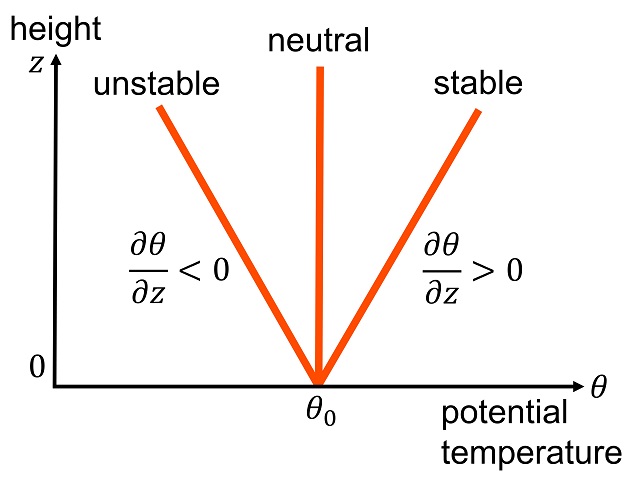 Potential temperature is a useful measure of the static stability of the unsaturated atmosphere. Under normal, stably stratified conditions, the potential temperature increases with height,
:
and vertical motions are suppressed. If the potential temperature decreases with height,
:
the atmosphere is unstable to vertical motions, and
Potential temperature is a useful measure of the static stability of the unsaturated atmosphere. Under normal, stably stratified conditions, the potential temperature increases with height,
:
and vertical motions are suppressed. If the potential temperature decreases with height,
:
the atmosphere is unstable to vertical motions, and
Eric Weisstein's World of Physics
at Wolfram Research {{Meteorological variables Atmospheric thermodynamics Meteorological quantities Physical oceanography
adiabatically
Adiabatic (from ''Gr.'' ἀ ''negative'' + διάβασις ''passage; transference'') refers to any process that occurs without heat transfer. This concept is used in many areas of physics and engineering. Notable examples are listed below.
A ...
brought to a standard reference pressure , usually . The potential temperature is denoted and, for a gas well-approximated as ideal, is given by
:
where is the current absolute temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
(in K) of the parcel, is the specific gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment pe ...
of air, and is the specific heat capacity
In thermodynamics, the specific heat capacity (symbol ) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as massic heat ...
at a constant pressure.
for air (meteorology). The reference point for potential temperature in the ocean is usually at the ocean's surface which has a water pressure of 0 dbar. The potential temperature in the ocean doesn't account for the varying heat capacities of seawater, therefore it is not a conservative measure of heat content. Graphical representation of potential temperature will always be less than the actual temperature line in a temperature vs depth graph.
Contexts
The concept of potential temperature applies to any stratified fluid. It is most frequently used in theatmospheric sciences
Atmospheric science is the study of the Earth's atmosphere and its various inner-working physical processes. Meteorology includes atmospheric chemistry and atmospheric physics with a major focus on weather forecasting. Climatology is the study ...
and oceanography
Oceanography (), also known as oceanology, sea science, ocean science, and marine science, is the scientific study of the ocean, including its physics, chemistry, biology, and geology.
It is an Earth science, which covers a wide range of to ...
. The reason that it is used
in both fields is that changes in pressure can result in warmer fluid residing under colder fluid – examples being dropping air temperature with altitude and increasing water temperature with depth in very deep ocean trenches and
within the ocean mixed layer
The oceanic or limnological mixed layer is a layer in which active turbulence has homogenized some range of depths. The surface mixed layer is a layer where this turbulence is generated by winds, surface heat fluxes, or processes such as evaporat ...
. When the potential temperature is used instead, these apparently unstable conditions vanish as a parcel of fluid is invariant along its isolines. In the oceans, the potential temperature referenced to the surface will be slightly less than the in-situ temperature (the temperature that a water volume has at the specific depth that the instrument measured it in) since the expansion due to reduction in pressure leads to cooling. The numeric difference between the in situ and potential temperature is almost always less than 1.5 degrees Celsius. However, it's important to use potential temperature when comparing temperatures of water from very different depths.
Comments
Potential temperature is a more dynamically important quantity than the actual temperature. This is because it is not affected by the physical lifting or sinking associated with flow over obstacles or large-scale atmospheric turbulence. A parcel of air moving over a small mountain will expand and cool as it ascends the slope, then compress and warm as it descends on the other side- but the potential temperature will not change in the absence of heating, cooling, evaporation, or condensation (processes that exclude these effects are referred to as dry adiabatic). Since parcels with the same potential temperature can be exchanged without work or heating being required, lines of constant potential temperature are natural flow pathways. Under almost all circumstances, potential temperature increases upwards in the atmosphere, unlike actual temperature which may increase or decrease. Potential temperature is conserved for all dry adiabatic processes, and as such is an important quantity in theplanetary boundary layer
In meteorology, the planetary boundary layer (PBL), also known as the atmospheric boundary layer (ABL) or peplosphere, is the lowest part of the atmosphere and its behaviour is directly influenced by its contact with a planetary surface. On Ea ...
(which is often very close to being dry adiabatic).
 Potential temperature is a useful measure of the static stability of the unsaturated atmosphere. Under normal, stably stratified conditions, the potential temperature increases with height,
:
and vertical motions are suppressed. If the potential temperature decreases with height,
:
the atmosphere is unstable to vertical motions, and
Potential temperature is a useful measure of the static stability of the unsaturated atmosphere. Under normal, stably stratified conditions, the potential temperature increases with height,
:
and vertical motions are suppressed. If the potential temperature decreases with height,
:
the atmosphere is unstable to vertical motions, and convection
Convection is single or Multiphase flow, multiphase fluid flow that occurs Spontaneous process, spontaneously through the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoy ...
is likely. Since convection acts to quickly mix the atmosphere and return to a stably stratified state, observations of decreasing potential temperature with height are uncommon, except while vigorous convection is underway or during periods of strong insolation
Solar irradiance is the power per unit area ( surface power density) received from the Sun in the form of electromagnetic radiation in the wavelength range of the measuring instrument.
Solar irradiance is measured in watts per square metre ...
. Situations in which the equivalent potential temperature decreases with height, indicating instability in saturated air, are much more common.
Since potential temperature is conserved under adiabatic or isentropic
An isentropic process is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in eng ...
air motions, in steady, adiabatic flow lines or surfaces of constant potential temperature act as streamlines or flow surfaces, respectively. This fact is used in isentropic analysis, a form of synoptic analysis which allows visualization of air motions and in particular analysis of large-scale vertical motion.
Potential temperature perturbations
The atmospheric boundary layer (ABL) potential temperature perturbation is defined as the difference between the potential temperature of the ABL and the potential temperature of the free atmosphere above the ABL. This value is called the potential temperature deficit in the case of a katabatic flow, because the surface will always be colder than the free atmosphere and the PT perturbation will be negative.Derivation
Theenthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
form of the first law of thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed b ...
can be written as:
:
where denotes the enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
change, the temperature, the change in entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
, the specific volume, and the pressure.
For adiabatic processes, the change in entropy is 0 and the 1st law simplifies to:
:
For approximately ideal gases, such as the dry air in the Earth's atmosphere, the equation of state
In physics and chemistry, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most mo ...
, can be substituted into the 1st law
yielding, after some rearrangement:
:
where the was used and both terms were divided by the product
Integrating yields:
:
and solving for , the temperature a parcel would acquire if moved adiabatically to the pressure level , you get:
:
Potential virtual temperature
The potential virtual temperature , defined by : is the theoretical potential temperature of the dry air which would have the same density as the humid air at a standard pressure P0. It is used as a practical substitute for density in buoyancy calculations. In this definition is the potential temperature, is the mixing ratio of water vapor, and is the mixing ratio of liquid water in the air.Related quantities
The Brunt–Väisälä frequency is a closely related quantity that uses potential temperature and is used extensively in investigations of atmospheric stability.See also
* Wet-bulb potential temperature *Atmospheric thermodynamics Atmospheric thermodynamics is the study of heat-to-Work (physics), work transformations (and their reverse) that take place in the Earth's atmosphere and manifest as weather or climate. Atmospheric thermodynamics use the laws of classical thermodyn ...
* Conservative temperature
* Equivalent potential temperature
References
Bibliography
* M K Yau and R.R. Rogers, ''Short Course in Cloud Physics, Third Edition'', published by Butterworth-Heinemann, January 1, 1989, 304 pages.External links
Eric Weisstein's World of Physics
at Wolfram Research {{Meteorological variables Atmospheric thermodynamics Meteorological quantities Physical oceanography