Potassium Peroxymonosulfate on:
[Wikipedia]
[Google]
[Amazon]
Potassium peroxymonosulfate is widely used as an
 Oxone converts
Oxone converts  Oxone is used in the production of some organic
Oxone is used in the production of some organic
oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
, for example, in pools and spas (usually referred to as monopersulfate or "MPS"). It is the potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
of peroxymonosulfuric acid
Peroxymonosulfuric acid, also known as persulfuric acid, peroxysulfuric acid is the inorganic compound with the formula . It is a white solid. It is a component of Caro's acid, which is a solution of peroxymonosulfuric acid in sulfuric acid cont ...
. Potassium peroxymonosulfate per se is rarely encountered. It is often confused with the triple salt , known as Oxone.
The standard electrode potential
In electrochemistry, standard electrode potential E^\ominus, or E^\ominus_, is the electrode potential (a measure of the reducing power of any element or compound) which the IUPAC "Gold Book" defines as ''"the value of the standard emf ( electrom ...
for potassium peroxymonosulfate is +1.81 V with a half reaction
In chemistry, a half reaction (or half-cell reaction) is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the r ...
generating the hydrogen sulfate ():
:
Oxone
Potassium peroxymonosulfate per se is a relatively obscure salt, but its derivative called Oxone is of commercial value. Oxone refers to the triple salt . As such about one third by weight is potassium peroxymonosulfate. Oxone has a longer shelf life than does potassium peroxymonosulfate. A white, water-soluble solid, Oxone loses <1% of its oxidizing power per month. Oxone, which is commercially available, is produced from peroxysulfuric acid, which is generated in situ by combiningoleum
Oleum (Latin ''oleum'', meaning oil), or fuming sulfuric acid, is a term referring to solutions of various compositions of sulfur trioxide in sulfuric acid, or sometimes more specifically to disulfuric acid (also known as pyrosulfuric acid).
Ol ...
and hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
. Careful neutralization of this solution with potassium hydroxide allows the crystallization of the triple salt.
Uses
Cleaning
Oxone is used widely for cleaning. It whitens dentures, oxidizes organic contaminants in swimming pools, and cleans chips for the manufacture of microelectronics.Organic oxidations
Oxone is a versatile oxidant in organic synthesis. It oxidizesaldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s to carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s; in the presence of alcoholic solvents, the ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s may be obtained. Internal alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s may be cleaved to two carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s (see below), while terminal alkenes may be epoxidized. Sulfides
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families of ...
give sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
s, tertiary amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s give amine oxide
In chemistry, an amine oxide, also known as an amine ''N''-oxide or simply ''N''-oxide, is a chemical compound that has the chemical formula . It contains a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substitue ...
s, and phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
s give phosphine oxide
Phosphine oxide is the inorganic compound with the formula H3PO. Although stable as a dilute gas, liquid or solid samples are unstable. Unlike many other compounds of the type POxHy, H3PO is rarely discussed and is not even mentioned in major so ...
s.
Further illustrative of the oxidative
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
power of this salt is the conversion of an acridine
Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups ...
derivative to the corresponding acridine-N-oxide
In chemistry, an amine oxide, also known as an amine ''N''-oxide or simply ''N''-oxide, is a chemical compound that has the chemical formula . It contains a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substitue ...
.
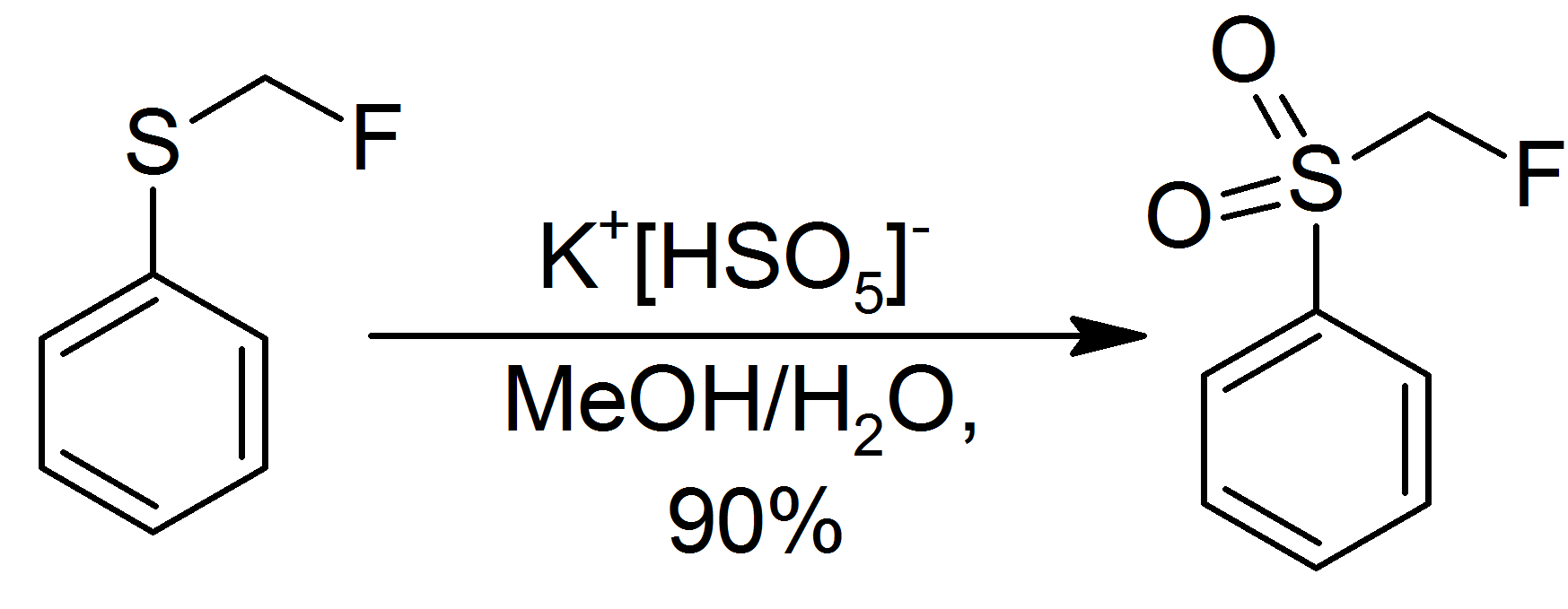
Oxone oxidizes sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
s to sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s and then to sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
s.
 Oxone converts
Oxone converts ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s to dioxirane
In chemistry, dioxirane (systematically named dioxacyclopropane, also known as methylene peroxide or peroxymethane) is an organic compound with formula . The molecule consists of a ring with one methylene and two oxygen atoms. It is of interes ...
s, which are used for diverse oxidations
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
in organic synthesis. The dominant reagent dimethyldioxirane
Dimethyldioxirane (DMDO) is the organic compound with the formula . It is the dioxirane derived from acetone and can be viewed as the monomer of acetone peroxide. It is a powerful selective oxidizing agent that finds some use in organic synthesis ...
(DMDO) forms upon treatment of acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
with oxone. Dioxiranes are versatile, especially for the epoxidation
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
of olefins
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of P ...
. Dioxiranes are also oxidize other unsaturated functionality, heteroatoms, and alkane C-H bonds.
 Oxone is used in the production of some organic
Oxone is used in the production of some organic periodinane Periodinanes also known as lambda, λ5-iodanes are organoiodine compounds with iodine in the +5 oxidation state. These compounds are described as hypervalency, hypervalent because the iodine center has more than 8 valence electrons.
Periodinane com ...
s, notably the oxidation of 2-iodobenzoic acid to 2-iodoxybenzoic acid
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is most often prepared from 2-iodobenzoic acid and a strong oxidant su ...
(IBX).
center, 300px, Oxidation of 2-iodobenzoic acid to IBX
Other uses
Oxone has been investigated for the delignification of wood.Ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
, sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
, and potassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
salts of are used in the plastics industry
The plastics industry manufactures polymer materials—commonly called plastics—and offers services in plastics important to a range of industries, including packaging, building and construction, electronics, aerospace, manufacturing and transpo ...
as radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical in ...
s for polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
. They are also used as etchants, oxidative desizing agents for textile fabrics, and for decolorizing and deodorizing oils.
References
{{Persulfates Persulfates Potassium compounds Oxidizing agents