Potassium nonahydridorhenate on:
[Wikipedia]
[Google]
[Amazon]
Potassium nonahydridorhenate(VII) is an
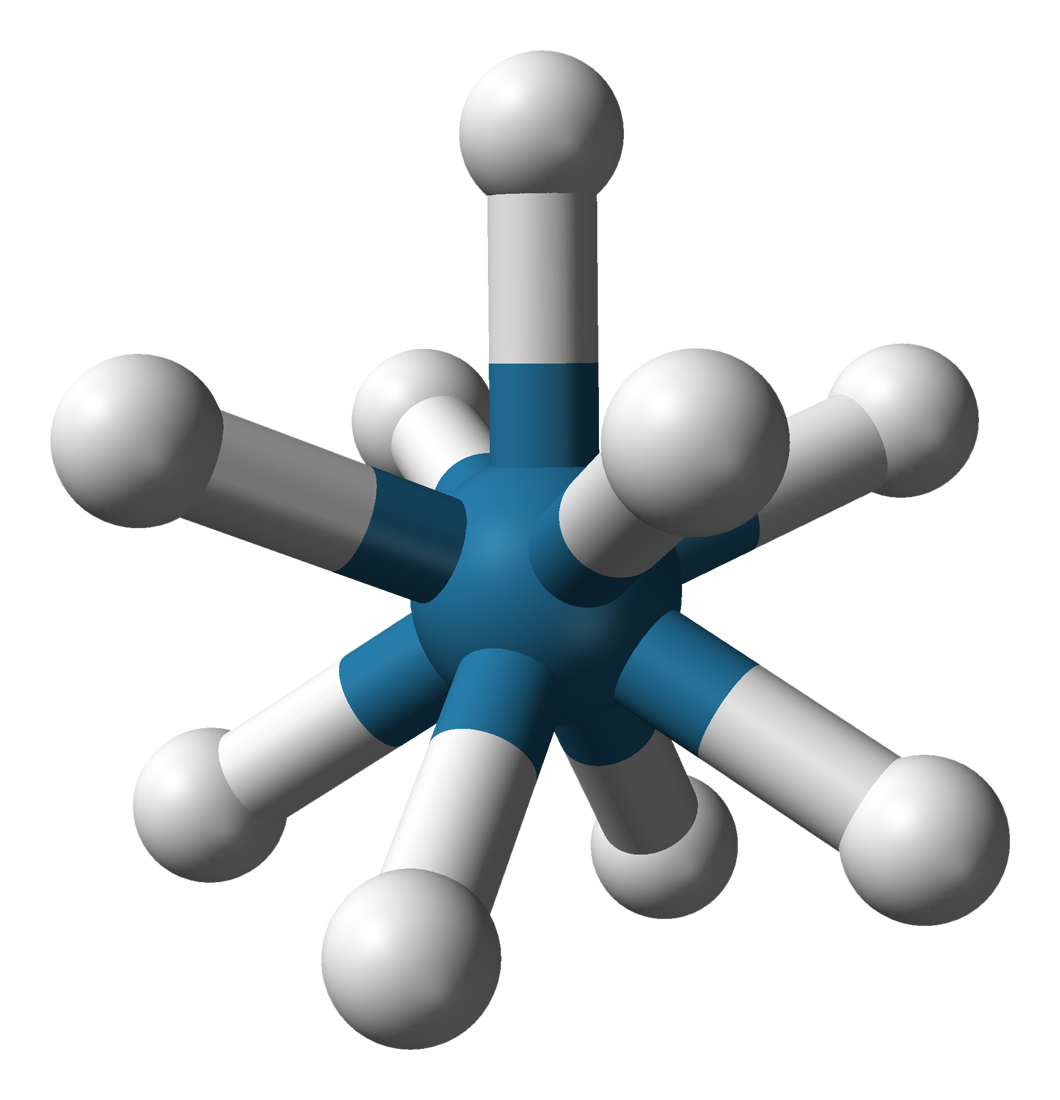 is an unusual example of a nonacoordinated
is an unusual example of a nonacoordinated
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
having the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
. This colourless salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
is soluble in water but only poorly soluble in most alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
. This salt contains the nonahydridorhenate(VII) anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
, , which is a rare example of a coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
bearing only hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ...
.
History
The study ofrhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
hydrides can be traced to the 1950s and included reports of the "rhenide" anion, supposedly . These reports led to a series of investigations by A. P. Ginsberg and coworkers on the products from the reduction of perrhenate The perrhenate ion is the anion with the formula , or a compound containing this ion. The perrhenate anion is tetrahedral, being similar in size and shape to perchlorate and the valence isoelectronic permanganate. The perrhenate anion is stable ove ...
.
The ''rhenide'' anion, , was based on the product of the reduction of perrhenate The perrhenate ion is the anion with the formula , or a compound containing this ion. The perrhenate anion is tetrahedral, being similar in size and shape to perchlorate and the valence isoelectronic permanganate. The perrhenate anion is stable ove ...
salts, such as the reduction of potassium perrhenate () by potassium metal. "Potassium rhenide" was shown to exist as a tetrahydrated complex, with the postulated chemical formula (potassium rhenide tetrahydrate). This compound exhibits strongly reducing properties, and slowly yields hydrogen gas when dissolved in water. The lithium and thallous salts were also reported. Later research, however, indicates that the "rhenide" ion is actually a hydridorhenate complex. "Potassium rhenide" was shown to be in fact the potassium nonahydridorhenate(VII), , containing the nonahydridorhenate(VII) anion, , in which the oxidation state of rhenium is actually +7. Other methods of reduction of perrhenate salts yield compounds containing other hydrido- complexes, including .
Structure, synthesis, and properties
 is an unusual example of a nonacoordinated
is an unusual example of a nonacoordinated complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
, its high coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion ...
being attributed to the small size of the hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
ligand and the high positive charge
Electric charge (symbol ''q'', sometimes ''Q'') is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative''. Like charges repel each other and ...
on the Re(VII) central atom. Its structure consists of a tricapped trigonal prism, as determined by neutron crystallography. The diamagnetic
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagn ...
sodium salt, like the analogous technetium
Technetium is a chemical element; it has Symbol (chemistry), symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. Technetium and promethium are the only radioactive elements whose neighbours in the sense ...
compound, is prepared by treating an ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
solution of sodium perrhenate, , with sodium metal
Sodium is a chemical element; it has symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope i ...
. Via cation exchange
Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of ch ...
, it can be converted to the corresponding tetraethylammonium
Tetraethylammonium (TEA) is a quaternary ammonium cation with the chemical formula , consisting of four ethyl groups (, denoted Et) attached to a central nitrogen atom. It is a counterion used in the research laboratory to prepare lipophilic salt ...
salt, (tetraethylammonium nonahydridorhenate(VII)).
Isostructural
Isostructural chemical compounds have similar chemical structures. " Isomorphous" when used in the relation to crystal structures is not synonymous: in addition to the same atomic connectivity that characterises isostructural compounds, isomorphous ...
with (nonahydridotechnetate(VII)), consists of a trigonal prism with Tc atom in the center and six hydrogen atoms at the corners. Three more hydrogen ligands define a triangle lying parallel to the base and crossing the prism in its center (see figure). Although those hydride ligands are not equivalent, their electronic structure is almost the same. The coordination number of 9 in this complex is the highest known for any rhenium complex.
Recent density functional theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
calculations on indicate that this dianion adopts the D3h⇌C4v⇌D3h pathway in gas phase
In the physical sciences, a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a ...
and solution, such interconversion originally proposed by Muetterties featuring a capped square antiprism structure as transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
has very low energy barrier
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
. In solid, intramolecular motions of include (1) circle-dance mechanism (resembling Matisse's painting ''Dance (II)'') and (2) three-arm turnstile
A turnstile (also called a gateline, baffle gate, automated gate, turn gate in some regions) is a form of gate which allows one person to pass at a time. A turnstile can be configured to enforce One-way traffic#One-way traffic of people, one-way ...
rotation.
References
{{Rhenium compounds Hydrido complexes Potassium compounds Rhenium compounds