Polyketone on:
[Wikipedia]
[Google]
[Amazon]
 Polyketones are a family of high-performance
Polyketones are a family of high-performance
 Polyketones are noted for having extremely low defects (double ethylene insertions or double carbonyl insertions, in red):
:
Polyketones are noted for having extremely low defects (double ethylene insertions or double carbonyl insertions, in red):
: The activation barrier to give double carbonyl insertions is very high, so it does not occur. Brookhart's mechanistic studies show that the concentration of the alkyl-ethylene palladium complex required to give double ethylene insertions is very low at any one point:
:
Additionally, the Gibbs energy of activation of the alkyl-ethylene insertion is ~ 3 kcal/mol higher than the corresponding activation barrier for the alkyl-carbon monoxide insertion. As a result, defects occur at an extremely low rate (~ 1 part per million). The industrially-relevant palladium- dppp catalyst has also been investigated.
The activation barrier to give double carbonyl insertions is very high, so it does not occur. Brookhart's mechanistic studies show that the concentration of the alkyl-ethylene palladium complex required to give double ethylene insertions is very low at any one point:
:
Additionally, the Gibbs energy of activation of the alkyl-ethylene insertion is ~ 3 kcal/mol higher than the corresponding activation barrier for the alkyl-carbon monoxide insertion. As a result, defects occur at an extremely low rate (~ 1 part per million). The industrially-relevant palladium- dppp catalyst has also been investigated.
 Whereas much effort has involved discrete palladium complexes, an example in the patent literature claims that a combination of
Whereas much effort has involved discrete palladium complexes, an example in the patent literature claims that a combination of
Macrogalleria
{{Authority control Organic polymers Thermoplastics
thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
polymers. The polar ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
groups in the polymer backbone of these materials gives rise to a strong attraction between polymer chains, which increases the material's melting point (255 °C for copolymer (carbon monoxide ethylene), 220 °C for terpolymer (carbon monoxide, ethylene, propylene). Trade names include Poketone, Carilon, Karilon, Akrotek, and Schulaketon. Such materials also tend to resist solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
s and have good mechanical properties. Unlike many other engineering plastic
Engineering plastics are a group of plastic materials that have better mechanical or thermal properties than the more widely used commodity plastics (such as polystyrene, polyvinyl chloride, polypropylene and polyethylene).
Engineering plastic ...
s, aliphatic polyketones such as Shell Chemicals
Shell Chemicals is the petrochemicals arm of Shell plc. The name "Shell Chemicals" refers to the nearly seventy companies engaged in chemicals businesses for Shell, which together make up one of the largest petrochemical producers in the world. T ...
' Carilon are relatively easy to synthesize and can be derived from inexpensive monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s. Carilon is made with a palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
(II) catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
from ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
and carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
. A small fraction of the ethylene is generally replaced with propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like o ...
to reduce the melting point somewhat. Shell Chemical commercially launched Carilon thermoplastic polymer in the U.S. in 1996, but discontinued it in 2000. Hyosung announced that they would launch production in 2015.
Industrially, the ethylene-carbon monoxide co-polymer is most significant. This polymer is synthesized either as a methanol slurry, or ''via'' a gas phase reaction with immobilized catalysts.
Polymerization mechanism
Initiation and termination
Where external initiation is not employed for the methanol system, initiation can take place ''via'' methanolysis of the palladium(II) precursor, giving either a methoxide or a hydride complex. Termination occurs also by methanolysis. Depending on the end of the growing polymer chain, this results in either an ester or a ketone end group, and regenerating the palladium methoxide or hydride catalysts respectively.Propagation
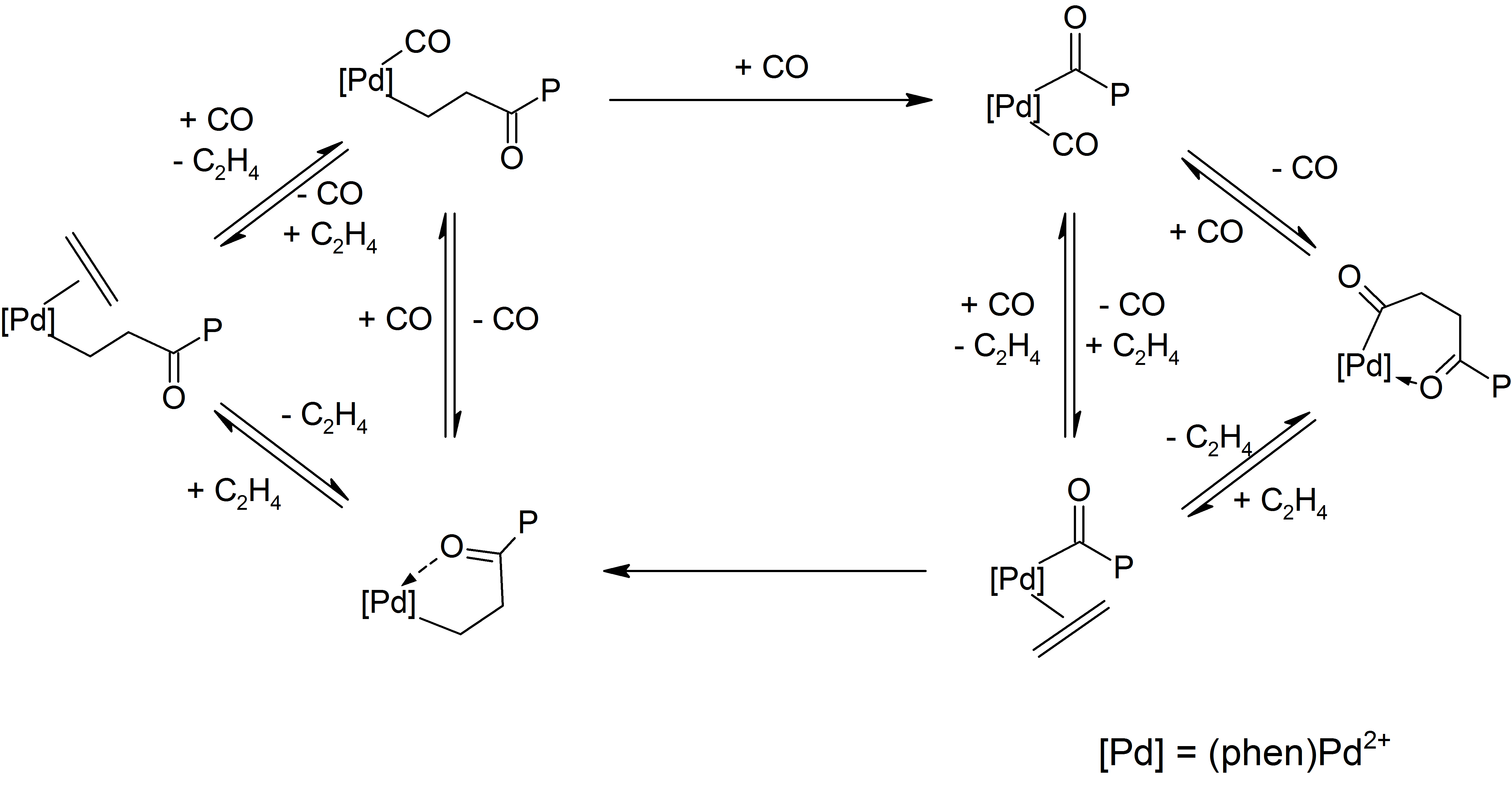
A mechanism for the propagation of this reaction using a palladium(II)- phenanthroline catalyst has been proposed by Maurice Brookhart: : Polyketones are noted for having extremely low defects (double ethylene insertions or double carbonyl insertions, in red):
:
Polyketones are noted for having extremely low defects (double ethylene insertions or double carbonyl insertions, in red):
: The activation barrier to give double carbonyl insertions is very high, so it does not occur. Brookhart's mechanistic studies show that the concentration of the alkyl-ethylene palladium complex required to give double ethylene insertions is very low at any one point:
:
Additionally, the Gibbs energy of activation of the alkyl-ethylene insertion is ~ 3 kcal/mol higher than the corresponding activation barrier for the alkyl-carbon monoxide insertion. As a result, defects occur at an extremely low rate (~ 1 part per million). The industrially-relevant palladium- dppp catalyst has also been investigated.
The activation barrier to give double carbonyl insertions is very high, so it does not occur. Brookhart's mechanistic studies show that the concentration of the alkyl-ethylene palladium complex required to give double ethylene insertions is very low at any one point:
:
Additionally, the Gibbs energy of activation of the alkyl-ethylene insertion is ~ 3 kcal/mol higher than the corresponding activation barrier for the alkyl-carbon monoxide insertion. As a result, defects occur at an extremely low rate (~ 1 part per million). The industrially-relevant palladium- dppp catalyst has also been investigated.
Importance of bidentate ligands
Where palladium(II) pre-catalysts bearing monodentate phosphine ligands are used in methanol, a relatively high fraction of methyl propionate is produced. In comparison, where chelatingdiphosphine
Diphosphane, or diphosphine, is an inorganic compound with the chemical formula . This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air.
Properties, ...
ligands are used, this side-product is absent. This observation is rationalized: the bis(phosphine) complex can undergo ''cis''-''trans'' isomerization to give the sterically favored ''trans'' isomer. The propionyl ligand is now ''trans''- to the open coordination site or ethylene ligand, and is unable to undergo migratory insertion. Instead, solvolysis by methanol occurs, which gives the undesired methyl propionate
Methyl propionate, also known as methyl propanoate, is an organic compound with the molecular formula . It is a colorless liquid with a fruity, rum-like odor.
Preparation
Methyl propionate can be prepared by esterification of propionic acid with ...
side-product.
: Whereas much effort has involved discrete palladium complexes, an example in the patent literature claims that a combination of
Whereas much effort has involved discrete palladium complexes, an example in the patent literature claims that a combination of Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
(aluminum, iron, or titanium halide) and a source of palladium (as a salt or the metal) is effective for making polyketone.Grant Proulx, (2000), "Preparation of olefin copolymers of sulfur dioxide or carbon monoxide", US Patent US006037442A
References
External links
Macrogalleria
{{Authority control Organic polymers Thermoplastics