Polyaspartate on:
[Wikipedia]
[Google]
[Amazon]
 Polyaspartic acid (PASA) is a
Polyaspartic acid (PASA) is a
 Many different routes lead to PASA. In the simplest and the oldest approach aspartic acid is heated to induce dehydration. In a subsequent step the resulting polysuccinimide is treated with aqueous
Many different routes lead to PASA. In the simplest and the oldest approach aspartic acid is heated to induce dehydration. In a subsequent step the resulting polysuccinimide is treated with aqueous
99–111
, isbn= 978-0-8412-3133-7 , chapter-url-access= registration , chapter-url= https://archive.org/details/hydrophilicpolym0000unse , url= https://archive.org/details/hydrophilicpolym0000unse/page/99 {{cite journal , first1= H. , last1= Pivcova , first2= V. , last2= Saudek , first3= J. , last3= Drobnik , first4= J. , last4= Vlasak , year= 1981 , title= NMR Study of Poly(aspartic acid). I. α-and β-Peptide Bonds in Poly(aspartic acid) Prepared by Thermal Polycondensation , journal= Biopolymers , volume= 20 , issue= 8 , pages=1605–1614 , doi= 10.1002/bip.1981.360200804 , s2cid= 85201969 {{cite journal , first1= Etso , last1= Kokufuta , first2= Shinnichiro , last2= Suzuki , first3= Kaoru , last3= Harad , year= 1978 , title= Temperature Effect on the Molecular Weight and the Optical Purity of Anhydropolyaspartic Acid Prepared by Thermal Polycondensation , journal= Bulletin of the Chemical Society of Japan , volume= 51 , issue= 5 , pages=1555–1556 , doi= 10.1246/bcsj.51.1555 , doi-access= {{cite journal , first1= Takeshi , last1= Nakato , first2= Atsushi , last2= Kusuno , first3= Toyoji , last3= Kakuchi , year= 2000 , title= Synthesis of poly(succinimide) by bulk polycondensation of L-aspartic acid with an acid catalyst , journal= Journal of Polymer Science Part A: Polymer Chemistry , volume= 38 , issue= 1 , pages=117–122 , doi= 10.1002/(SICI)1099-0518(20000101)38:1<117::AID-POLA15>3.0.CO;2-F , bibcode= 2000JPoSA..38..117N , doi-access= free {{cite journal , first1= Yaquan , last1= Wang , first2= Yongjiang , last2= Hou , first3= Gang , last3= Ruan , first4= Ming , last4= Pan , first5= Tengfei , last5= Liu , year= 2003 , title= Study on the polymerization of aspartic acid catalyzed by phosphoric acid , journal= Journal of Macromolecular Science-Pure and Applied Chemistry , volume= A40 , issue= 3 , pages=293–307 , doi= 10.1081/MA-120018116 , s2cid= 85163135 {{cite journal , first1= Vanga S. , last1= Rao , first2= Philippe , last2= Lapointe , first3= Donald N. , last3= McGregor , year= 1993 , title= Temperature Effect on the Molecular Weight and the Optical Purity of Anhydropolyaspartic Acid Prepared by Thermal Polycondensation , journal= Makromolekulare Chemie-Macromolecular Chemistry and Physics , volume= 194 , issue= 4 , pages=1095–1104 , doi= 10.1002/macp.1993.021940405 {{cite journal , first1= Yasuyuki , last1= Soeda , first2= Kazunobu , last2= Toshima , first3= Shuichi , last3= Matsuma , year= 2003 , title= Sustainable enzymatic preparation of polyaspartate using a bacterial protease , journal= Biomacromolecules , volume= 4 , issue= 2 , pages=193–203 , doi= 10.1021/bm0200534 , pmid= 12625712 {{cite journal , first1= V. , last1= Saudek , first2= H. , last2= Pivcova , first3= J. , last3= Drobnik , year= 1981 , title= NMR Study of Poly(aspartic acid). II. α-and β-Peptide Bonds in Poly(aspartic acid) Prepared by Common Methods , journal= Biopolymers , volume= 20 , issue= 8 , pages=1615–1623 , doi= 10.1002/bip.1981.360200805 , s2cid= 84203387 {{cite patent , country = US , number = 5468838 , status = patent , title = Polysuccinimide, polyaspartic acid and their salts are prepared by reaction of maleic anhydride and ammonia, polycondensation of the resulting product in the presence of a solubilizing agent and, if appropriate, hydrolysis. , pubdate = 1995-11-21 , fdate = 1994-03-14 , invent1 = Boehmke, Gunter , invent2 = Schmitz, Gerd , assign1 = Bayer AG {{Cite journal , last1 = Gross , first1 = Richard A. , last2 = Kalra , first2 = Bhanu , year = 2002 , title = Biodegradable Polymers for the Environment , journal = Science , volume = 297 , issue = 5582 , pages = 803–807 , doi = 10.1126/science.297.5582.803 , pmid = 12161646 , bibcode=2002Sci...297..803G, url = https://zenodo.org/record/1231185 {{Cite journal , first1= David , last1= Hasson , first2= Hilla , last2= Shemer , first3= Alexander , last3= Sher , year= 2011 , title= State of the Art of Friendly "Green" Scale Control Inhibitors: A Review Article , journal= Industrial & Engineering Chemistry Research , volume= 50 , issue= 12 , pages=7601–7607 , doi= 10.1021/ie200370v {{Cite journal , first1= M. J. , last1= Zahuriaan-Mehr , first2= A. , last2= Pourjavadi , first3= H. , last3= Salimi , first4= M. , last4= Kurdtabar , year= 2009 , title= Protein- and homo poly(amino acid)-based hydrogels with super-swelling properties , journal= Polymers for Advanced Technologies , volume= 20 , issue= 8 , pages=655–671 , doi= 10.1002/pat.1395 {{Cite journal , last1 = Thombre , first1 = Sunita M. , last2 = Sarwade , first2 = Bhimaro D. , year = 2005 , title = Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review , journal = Journal of Macromolecular Science, Part A , volume = 42 , issue = 9 , pages = 1299–1315 , url = http://www.informaworld.com/index/718581646.pdf , doi = 10.1080/10601320500189604 , s2cid = 94818855 , 2 Polyamides Polyelectrolytes Chelating agents
 Polyaspartic acid (PASA) is a
Polyaspartic acid (PASA) is a biodegradable
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegrada ...
, water-soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
condensation polymer
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Natural proteins as well as s ...
based on the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protei ...
. It is a biodegradable replacement for water softeners and related applications. PASA can be chemically crosslinked with a wide variety of methods to yield PASA hydrogel
A hydrogel is a Phase (matter), biphasic material, a mixture of Porosity, porous and Permeation, permeable solids and at least 10% of water or other interstitial fluid. The solid phase is a water Solubility, insoluble three dimensional network ...
s. The resulting hydrogels are pH-sensitive such that under acidic conditions, they shrink, while the swelling capacity increases under alkaline conditions.
Sodium polyaspartate is a sodium salt of polyaspartic acid.
In nature, PASA has been found in as fragments of larger proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, re ...
with length up to 50 amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
, but as of 2004 had not been isolated as a pure homo polymeric material from any natural source. The first isolation of synthetic oligomeric sodium polyaspartate
Sodium is a chemical element; it has symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope i ...
, obtained by thermal polycondensation
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Natural proteins as well as s ...
of aspartic acid, was reported by Hugo Schiff
Hugo (Ugo) Schiff (26 April 1834 – 8 September 1915) was an Italian naturalized chemist. The son of a Jewish businessman and brother of the physiologist Moritz Schiff, Hugo Schiff was German by nationality. He discovered Schiff bases and o ...
in late 19th century. Later it was proposed that thermal polymerization process leads through polysuccinimide intermediate. Polyaspartic acid is produced industrially in both the acid form and as the sodium salt.
Properties and structure
Due to presence ofcarboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ...
ic groups it is polyelectrolyte
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are t ...
with anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
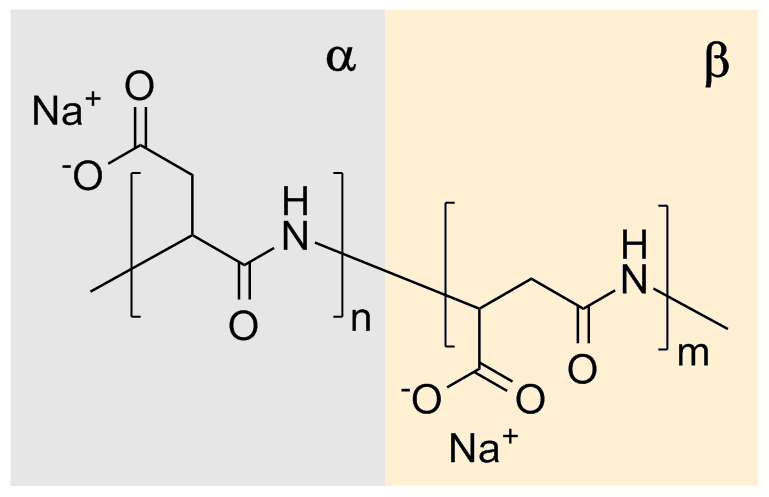
ic character. Naturally occurring PASA fragments consists of α,-linked L-aspartatic acid. In contrast, the repeating unit of synthetic polyaspartic acid may exist in four isomeric forms, depending on the stereochemistry of starting material (D- and L-aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protei ...
) and synthetic procedure leading to α and β links. Due to the protein-like backbone (presence of amide bond in the backbone), PASA has suitable biodegradability
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegrada ...
.
Synthesis
 Many different routes lead to PASA. In the simplest and the oldest approach aspartic acid is heated to induce dehydration. In a subsequent step the resulting polysuccinimide is treated with aqueous
Many different routes lead to PASA. In the simplest and the oldest approach aspartic acid is heated to induce dehydration. In a subsequent step the resulting polysuccinimide is treated with aqueous sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
, which yields partial opening of the succinimide
Succinimide is an organic compound with the formula (CH2)2(CO)2NH. This white solid is used in a variety of organic syntheses, as well as in some industrial silver plating processes. The compound is classified as a cyclic imide. It may be prepared ...
rings. In this process sodium-DL-(α,β)-poly(aspartate) with 30% α-linkages and 70% β-linkages randomly distributed along the polymer chain, and racemized chiral center of aspartic acid is produced. There were many catalysts reported for improving thermal polymerization method. Main benefits from their application is increasing of the conversion rate and higher molecular weight of the product.
Polyaspartic acid can also be synthesized by polymerization of maleic anhydride
Maleic anhydride is an organic compound with the formula . It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.
Str ...
in presence of ammonium hydroxide
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although ...
.
High control over repeating unit isomers can be achieved by polymerization of N-carboxyanhydride (NCA) derivatives, by polymerization of aspartic acid esters or by application of enzyme catalyzed reaction. Pure homopolymers, D- or L-PASA with α- or β-links only, can be synthesized using those methods.
The polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
reaction is an example of a step-growth polymerization
In polymer chemistry, step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Ma ...
to a polyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made throug ...
. In one procedure, aspartic acid polymerizes at 180 °C
The degree Celsius is the unit of temperature on the Celsius temperature scale "Celsius temperature scale, also called centigrade temperature scale, scale based on 0 ° for the melting point of water and 100 ° for the boiling point ...
concomitant with dehydration and the formation of a poly(succinimide
Succinimide is an organic compound with the formula (CH2)2(CO)2NH. This white solid is used in a variety of organic syntheses, as well as in some industrial silver plating processes. The compound is classified as a cyclic imide. It may be prepared ...
). The resulting polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
reacts with aqueous sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
, which hydrolyzes
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
one of the two amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
bonds of the succinimide ring to form a sodium carboxylate. The remaining amide bond is thus the linkage between successive aspartate residues. Each aspartate residue is identified as α or β according to which carbonyl of it is part of the polymer chain. The α form has one carbon in the backbone in addition to the carbonyl itself (and a two-carbon sidechain) whereas the β form has two carbons in the backbone in addition to the carbonyl itself (and a one-carbon sidechain). This reaction gives a sodium poly(aspartate) composed of approximately 30% α-linkages and 70% β-linkages.
Applications
Polyaspartic acid and its derivatives are biodegradable alternatives to traditional polyanionic materials, in particularpolyacrylic acid
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2−CHCO2H)''n''. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially ...
. PASA has ability to inhibit deposition of calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
, calcium sulfate
Calcium sulfate (or calcium sulphate) is an inorganic salt with the chemical formula . It occurs in several hydrated forms; the anhydrous state (known as anhydrite) is a white crystalline solid often found in evaporite deposits. Its dihydrate ...
, barium sulfate
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula Ba SO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs in nature as the mineral barite, which is the main commercial source of ...
, and calcium phosphate and can be used as an antiscaling agent in cooling water systems, water desalination processes, and waste water treatment operations. In addition and due to its ability to chelate
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
metal ions, it provides corrosion inhibition. It can also be used as biodegradable detergent and dispersant for various applications.
PASA also has a variety of biomedical
Biomedicine (also referred to as Western medicine, mainstream medicine or conventional medicine)
applications. Its high affinity with calcium has been exploited for targeting various forms of drug-containing carriers to the bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, ...
. The main component of bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, ...
is hydroxyapatite
Hydroxyapatite (International Mineralogical Association, IMA name: hydroxylapatite) (Hap, HAp, or HA) is a naturally occurring mineral form of calcium apatite with the Chemical formula, formula , often written to denote that the Crystal struc ...
(ca. 70%) (mineralized calcium phosphate
The term calcium phosphate refers to a family of materials and minerals containing calcium ions (Ca2+) together with inorganic phosphate anions. Some so-called calcium phosphates contain oxide and hydroxide as well. Calcium phosphates are white ...
). Apart from bone targeting, PASA has been modified for other biomedical applications such as drug delivery
Drug delivery involves various methods and technologies designed to transport pharmaceutical compounds to their target sites helping therapeutic effect. It involves principles related to drug preparation, route of administration, site-specif ...
, surface coating, DNA delivery, mucoadhesion,
and beyond.
As it can be synthesized in an environmentally friendly way and is biodegradable
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegrada ...
, polyaspartate is a potential green
Green is the color between cyan and yellow on the visible spectrum. It is evoked by light which has a dominant wavelength of roughly 495570 nm. In subtractive color systems, used in painting and color printing, it is created by a com ...
alternative to several materials such as sodium polyacrylate
Sodium polyacrylate (ACR, ASAP, or PAAS), also known as waterlock, is a sodium salt of polyacrylic acid with the chemical formula ��CH2−CH(CO2Na)− and has broad applications in consumer products. This Superabsorbent polymer, super-absorb ...
used in disposable diaper
A diaper (, North American English) or a nappy (British English, Australian English, Hiberno-English) is a type of underwear that allows the wearer to urinate or defecate without using a toilet, by absorbing or containing waste products to p ...
s and agriculture. It can act as a super-swelling material in diaper
A diaper (, North American English) or a nappy (British English, Australian English, Hiberno-English) is a type of underwear that allows the wearer to urinate or defecate without using a toilet, by absorbing or containing waste products to p ...
s, feminine hygiene
Feminine hygiene products are personal care products used for women's hygiene during menstruation, vaginal discharge, or other bodily functions related to the vulva and vagina. Products that are used during menstruation may also be called menstru ...
products, and food packaging
Food packaging is a packaging system specifically designed for food and represents one of the most important aspects among the processes involved in the food industry, as it provides protection from chemical, biological and physical alterations ...
. The level of water uptake which is inversely related to the mechanical properties of the hydrogel can be tuned by changing the crosslinking density.In addition to its industrial uses, solid-state NMR studies have shown that poly‑aspartate can integrate into amorphous calcium carbonate (ACC) nanoparticles, adopting α‑helix conformations that significantly stabilize the ACC phase and delay its crystallization. Moreover, NMR relaxation data reveal that structural water molecules within ACC undergo millisecond-timescale 180° flips, suggesting that dynamic hydration plays a crucial role in the stabilization mechanism.
See also
* Polyaspartic estersReferences
{{Reflist, refs= {{cite book , first1= Kirt W. , last1= Rusenko , first2= Julie E. , last2= Donachy , first3= A. P. , last3= Wheeler , editor1-first= C. Steven , editor1-last= Sikes , editor2-first= A. P. , editor2-last= Wheeler , year= 1991 , title= Surface Reactive Peptides and Polymers , doi= 10.1021/bk-1991-0444.ch008 , series = ACS Symposium Series , volume = 444 , chapter = Purification And Characterization Of A Shell Matrix Phosphoprotein From The American Oyster , publisher= ACS , pages= 107–124 , isbn= 978-0-8412-1886-4 {{cite book , first1= Winfried , last1= Joentgen , first2= Nikolaus , last2= Müller , first3= Alfred , last3= Mitschker , first4= Holger , last4= Schmidt , editor1-first= Stephen , editor1-last= Fahnestock , editor2-first= Alexander , editor2-last= Steinbüchel , title= Polyamides and Complex Proteinaceous Materials I , volume =7 , series = Biopolymers , chapter-url= http://www.wiley-vch.de/books/biopoly/con_v07.html , year= 2004 , publisher= Wiley-VCH , pages= 175–179 , chapter= Polyaspartic acids , isbn= 978-3-527-30222-2 {{cite journal , first1= Hugo , last1= Schiff , year= 1897 , title= Ueber Polyaspartsäuren , journal= Ber. Dtsch. Chem. Ges. , volume= 30 , issue= 3 , language= german , pages= 2449–2459 , doi= 10.1002/cber.18970300316 {{cite journal , first1= J. , last1= Kovács , first2= I. , last2= Könyves , first3=Á. , last3= Pusztai , year= 1953 , title= Darstellung von Polyasparaginsäuren (Polyaspartsäuren) aus dem thermischen Autokondensationsprodukt der Asparaginsäure , journal= Experientia , volume= 9 , issue= 12 , language= german , pages=459–460 , doi= 10.1007/BF02165821 , pmid= 13127859 , s2cid= 40153017 {{cite journal , first1= G. D. , last1= Bennett , title= A Green Polymerization of Aspartic Acid for the Undergraduate Organic Laboratory , journal= Journal of Chemical Education , year= 2005 , volume= 82 , issue= 9 , pages= 1380–1381 , doi= 10.1021/ed082p1380 , bibcode = 2005JChEd..82.1380B {{cite journal , first1= J. , last1= Kovács , first2= I. , last2= Könyves , year= 1954 , title= Uber DL-α,β-Polyasparaginsaure , journal= Naturwissenschaften , volume= 41 , issue= 14 , language= german , page=333 , doi= 10.1007/BF00644501 , bibcode = 1954NW.....41..333K , s2cid= 33648417 {{cite book , first1= Kim C. , last1= Low , first2= A. P. , last2= Wheeler , first3= Larry P. , last3= Koskan , editor1-first= J. Edward , editor1-last= Glass , year= 1996 , title= Hydrophilic Polymers , doi= 10.1021/ba-1996-0248.ch006 , series= Advances in Chemistry , volume= 248 , chapter= 6: Commercial Poly(aspartic acid) and Its Uses , publisher= ACS , pages99–111
, isbn= 978-0-8412-3133-7 , chapter-url-access= registration , chapter-url= https://archive.org/details/hydrophilicpolym0000unse , url= https://archive.org/details/hydrophilicpolym0000unse/page/99 {{cite journal , first1= H. , last1= Pivcova , first2= V. , last2= Saudek , first3= J. , last3= Drobnik , first4= J. , last4= Vlasak , year= 1981 , title= NMR Study of Poly(aspartic acid). I. α-and β-Peptide Bonds in Poly(aspartic acid) Prepared by Thermal Polycondensation , journal= Biopolymers , volume= 20 , issue= 8 , pages=1605–1614 , doi= 10.1002/bip.1981.360200804 , s2cid= 85201969 {{cite journal , first1= Etso , last1= Kokufuta , first2= Shinnichiro , last2= Suzuki , first3= Kaoru , last3= Harad , year= 1978 , title= Temperature Effect on the Molecular Weight and the Optical Purity of Anhydropolyaspartic Acid Prepared by Thermal Polycondensation , journal= Bulletin of the Chemical Society of Japan , volume= 51 , issue= 5 , pages=1555–1556 , doi= 10.1246/bcsj.51.1555 , doi-access= {{cite journal , first1= Takeshi , last1= Nakato , first2= Atsushi , last2= Kusuno , first3= Toyoji , last3= Kakuchi , year= 2000 , title= Synthesis of poly(succinimide) by bulk polycondensation of L-aspartic acid with an acid catalyst , journal= Journal of Polymer Science Part A: Polymer Chemistry , volume= 38 , issue= 1 , pages=117–122 , doi= 10.1002/(SICI)1099-0518(20000101)38:1<117::AID-POLA15>3.0.CO;2-F , bibcode= 2000JPoSA..38..117N , doi-access= free {{cite journal , first1= Yaquan , last1= Wang , first2= Yongjiang , last2= Hou , first3= Gang , last3= Ruan , first4= Ming , last4= Pan , first5= Tengfei , last5= Liu , year= 2003 , title= Study on the polymerization of aspartic acid catalyzed by phosphoric acid , journal= Journal of Macromolecular Science-Pure and Applied Chemistry , volume= A40 , issue= 3 , pages=293–307 , doi= 10.1081/MA-120018116 , s2cid= 85163135 {{cite journal , first1= Vanga S. , last1= Rao , first2= Philippe , last2= Lapointe , first3= Donald N. , last3= McGregor , year= 1993 , title= Temperature Effect on the Molecular Weight and the Optical Purity of Anhydropolyaspartic Acid Prepared by Thermal Polycondensation , journal= Makromolekulare Chemie-Macromolecular Chemistry and Physics , volume= 194 , issue= 4 , pages=1095–1104 , doi= 10.1002/macp.1993.021940405 {{cite journal , first1= Yasuyuki , last1= Soeda , first2= Kazunobu , last2= Toshima , first3= Shuichi , last3= Matsuma , year= 2003 , title= Sustainable enzymatic preparation of polyaspartate using a bacterial protease , journal= Biomacromolecules , volume= 4 , issue= 2 , pages=193–203 , doi= 10.1021/bm0200534 , pmid= 12625712 {{cite journal , first1= V. , last1= Saudek , first2= H. , last2= Pivcova , first3= J. , last3= Drobnik , year= 1981 , title= NMR Study of Poly(aspartic acid). II. α-and β-Peptide Bonds in Poly(aspartic acid) Prepared by Common Methods , journal= Biopolymers , volume= 20 , issue= 8 , pages=1615–1623 , doi= 10.1002/bip.1981.360200805 , s2cid= 84203387 {{cite patent , country = US , number = 5468838 , status = patent , title = Polysuccinimide, polyaspartic acid and their salts are prepared by reaction of maleic anhydride and ammonia, polycondensation of the resulting product in the presence of a solubilizing agent and, if appropriate, hydrolysis. , pubdate = 1995-11-21 , fdate = 1994-03-14 , invent1 = Boehmke, Gunter , invent2 = Schmitz, Gerd , assign1 = Bayer AG {{Cite journal , last1 = Gross , first1 = Richard A. , last2 = Kalra , first2 = Bhanu , year = 2002 , title = Biodegradable Polymers for the Environment , journal = Science , volume = 297 , issue = 5582 , pages = 803–807 , doi = 10.1126/science.297.5582.803 , pmid = 12161646 , bibcode=2002Sci...297..803G, url = https://zenodo.org/record/1231185 {{Cite journal , first1= David , last1= Hasson , first2= Hilla , last2= Shemer , first3= Alexander , last3= Sher , year= 2011 , title= State of the Art of Friendly "Green" Scale Control Inhibitors: A Review Article , journal= Industrial & Engineering Chemistry Research , volume= 50 , issue= 12 , pages=7601–7607 , doi= 10.1021/ie200370v {{Cite journal , first1= M. J. , last1= Zahuriaan-Mehr , first2= A. , last2= Pourjavadi , first3= H. , last3= Salimi , first4= M. , last4= Kurdtabar , year= 2009 , title= Protein- and homo poly(amino acid)-based hydrogels with super-swelling properties , journal= Polymers for Advanced Technologies , volume= 20 , issue= 8 , pages=655–671 , doi= 10.1002/pat.1395 {{Cite journal , last1 = Thombre , first1 = Sunita M. , last2 = Sarwade , first2 = Bhimaro D. , year = 2005 , title = Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review , journal = Journal of Macromolecular Science, Part A , volume = 42 , issue = 9 , pages = 1299–1315 , url = http://www.informaworld.com/index/718581646.pdf , doi = 10.1080/10601320500189604 , s2cid = 94818855 , 2 Polyamides Polyelectrolytes Chelating agents