pi stacking on:
[Wikipedia]
[Google]
[Amazon]
In
 A combination of tetracyanoquinodimethane (TCNQ) and tetrathiafulvalene (TTF) forms a strong charge-transfer complex referred to as ''TTF-TCNQ''. The solid shows almost metallic electrical conductance. In a TTF-TCNQ crystal, TTF and TCNQ molecules are arranged independently in separate parallel-aligned stacks, and an electron transfer occurs from donor (TTF) to acceptor (TCNQ) stacks.
A combination of tetracyanoquinodimethane (TCNQ) and tetrathiafulvalene (TTF) forms a strong charge-transfer complex referred to as ''TTF-TCNQ''. The solid shows almost metallic electrical conductance. In a TTF-TCNQ crystal, TTF and TCNQ molecules are arranged independently in separate parallel-aligned stacks, and an electron transfer occurs from donor (TTF) to acceptor (TCNQ) stacks.

 π–π interactions play a role in
π–π interactions play a role in
Larry Wolf (2011): π-π (π-Stacking) interactions: origin and modulation
{{Chemical bonds Organic chemistry Chemical bonding Supramolecular chemistry
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, stacking refers to superposition of molecules or atomic sheets owing to attractive interactions between these molecules or sheets.
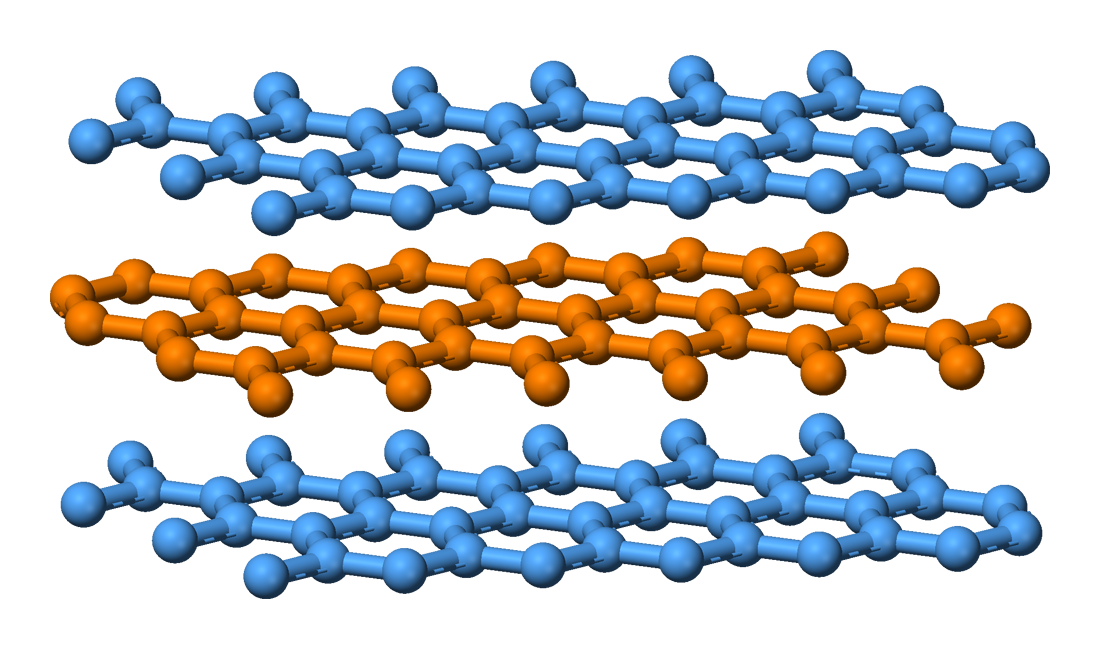
Metal dichalcogenide compounds
Metal dichalcogenides have the formula ME2, where M = a transition metal and E = S, Se, Te. In terms of their electronic structures, these compounds are usually viewed as derivatives of M4+. They adopt stacked structures, which is relevant to their ability to undergo intercalation, e.g. bylithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
, and their lubricating properties. The corresponding diselenides and even ditellurides are known, e.g., TiSe2, MoSe2, and WSe2.
Charge transfer salts
 A combination of tetracyanoquinodimethane (TCNQ) and tetrathiafulvalene (TTF) forms a strong charge-transfer complex referred to as ''TTF-TCNQ''. The solid shows almost metallic electrical conductance. In a TTF-TCNQ crystal, TTF and TCNQ molecules are arranged independently in separate parallel-aligned stacks, and an electron transfer occurs from donor (TTF) to acceptor (TCNQ) stacks.
A combination of tetracyanoquinodimethane (TCNQ) and tetrathiafulvalene (TTF) forms a strong charge-transfer complex referred to as ''TTF-TCNQ''. The solid shows almost metallic electrical conductance. In a TTF-TCNQ crystal, TTF and TCNQ molecules are arranged independently in separate parallel-aligned stacks, and an electron transfer occurs from donor (TTF) to acceptor (TCNQ) stacks.
Graphite

Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
consists of stacked sheets of covalently bonded carbon. The individual layers are called graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
. In each layer, each carbon atom is bonded to three other atoms forming a continuous layer of sp2 bonded carbon hexagons, like a honeycomb lattice with a bond length of 0.142 nm, and the distance between planes is 0.335 nm. Bonding between layers is relatively weak van der Waals bonds, which allows the graphene-like layers to be easily separated and to glide past each other. Electrical conductivity perpendicular to the layers is consequently about 1000 times lower.
Linear chain compounds
Linear chain compounds are materials composed of stacked arrays of metal-metal bonded molecules or ions. Such materials exhibitanisotropic
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit ver ...
electrical conductivity. One example is (acac = acetylacetonate, which stack with distances of about 326 pm. Classic examples include Krogmann's salt and Magnus's green salt.

Counterexample: benzene dimer and related species
π–π stacking is a noncovalent interaction between thepi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
s of aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
rings. Such "sandwich interactions" are however generally electrostatic
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges.
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word (), mean ...
ally repulsive. What is more commonly observed are either a staggered stacking (parallel displaced) or pi-teeing (perpendicular T-shaped) interaction both of which are electrostatic attractive. For example, the most commonly observed interactions between aromatic rings of amino acid residues in proteins is a staggered stacked followed by a perpendicular orientation. Sandwiched orientations are relatively rare. Pi stacking is repulsive as it places carbon atoms with partial negative charges from one ring on top of other partial negatively charged carbon atoms from the second ring and hydrogen atoms with partial positive charges on top of other hydrogen atoms that likewise carry partial positive charges.
 π–π interactions play a role in
π–π interactions play a role in supramolecular chemistry
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from w ...
, specifically the synthesis of catenane. The major challenge for the synthesis of catenane is to interlock molecules in a controlled fashion. Attractive π–π interactions exist between electron-rich benzene derivatives and electron-poor pyridinium rings. atanene was synthesized by treating bis(pyridinium) (A), bisparaphenylene-34-crown-10 (B), and 1, 4-bis(bromomethyl)benzene (C) (Fig. 2). The π–π interaction between A and B directed the formation of an interlocked template intermediate that was further cyclized by substitution reaction with compound C to generate the atenane product.
See also
* Noncovalent interaction *Dispersion (chemistry)
A dispersion is a system in which distributed particles of one material are dispersed in a continuous phase of another material. The two phases may be in the same or different states of matter.
Dispersions are classified in a number of diff ...
* Cation–pi interaction
* Intercalation (biochemistry)
In biochemistry, intercalation is the insertion of molecules between the planar bases of deoxyribonucleic acid (DNA). This process is used as a method for analyzing DNA and it is also the basis of certain kinds of poisoning.
There are several ...
* Intercalation (chemistry)
Intercalation is the reversible inclusion or insertion of a molecule (or ion) into layered materials with layered structures. Examples are found in graphite and transition metal dichalcogenides.
:
Examples Graphite
One famous intercalation hos ...
References
External links
*Larry Wolf (2011): π-π (π-Stacking) interactions: origin and modulation
{{Chemical bonds Organic chemistry Chemical bonding Supramolecular chemistry