Pi Interactions on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system can interact with a metal (cationic or neutral), an anion, another molecule and even another π system. Non-covalent interactions involving π systems are pivotal to biological events such as protein-ligand recognition.
 * Aromatic–aromatic interactions (π stacking): involves interactions of aromatic molecules with each other.
**Arene–perfluoroarene interaction: electron-rich benzene ring interacts with electron-poor
* Aromatic–aromatic interactions (π stacking): involves interactions of aromatic molecules with each other.
**Arene–perfluoroarene interaction: electron-rich benzene ring interacts with electron-poor  *π donor–acceptor interactions: interaction between low energy empty orbital (acceptor) and a high-energy filled orbital (donor).
*π donor–acceptor interactions: interaction between low energy empty orbital (acceptor) and a high-energy filled orbital (donor).
 *Anion–π interactions: interaction of anion with π system
* Cation–π interactions: interaction of a cation with a π system
*C–H–π interactions: interaction of C-H with π system: These interactions are well studied using experimental as well as computational techniques.
*Anion–π interactions: interaction of anion with π system
* Cation–π interactions: interaction of a cation with a π system
*C–H–π interactions: interaction of C-H with π system: These interactions are well studied using experimental as well as computational techniques.

 Allyl–π
Allyl–π

 π-effects have an important contribution to biological systems since they provide a significant amount of binding enthalpy. Neurotransmitters produce most of their biological effect by binding to the active site of a protein receptor. Pioneering work of Dennis A. Dougherty is a proof that such kind of binding stabilization is the effect of cation-π interactions of the
π-effects have an important contribution to biological systems since they provide a significant amount of binding enthalpy. Neurotransmitters produce most of their biological effect by binding to the active site of a protein receptor. Pioneering work of Dennis A. Dougherty is a proof that such kind of binding stabilization is the effect of cation-π interactions of the
 systems are important building blocks in
systems are important building blocks in \pi - \pi , CH-\pi and \pi -cation interactions are widely used in supramolecular assembly and \pi-\pi concerns the direct interactions between two -systems; and cation-\pi interaction arises from the electrostatic interaction of a cation with the face of the -system. Unlike these two interactions, the CH-\pi interaction arises mainly from charge transfer between the C–H orbital and the -system.
Types
The most common types of π-interactions involve: *Metal–π interactions: involves interaction of a metal and the face of a π system, the metal can be a cation (known as cation–π interactions) or neutral *Polar–π interactions: involves interaction of a polar molecule and quadrupole moment a π system.hexafluorobenzene
Hexafluorobenzene, HFB, , or perfluorobenzene is an organic, aromatic compound. In this derivative of benzene all hydrogen atoms have been replaced by fluorine atoms. The technical uses of the compound are limited, although it is recommended as ...
.
Metal–π interactions
Metal–π interactions play a major role inorganometallics
''Organometallics'' is a biweekly journal published by the American Chemical Society. Its area of focus is organometallic and organometalloid chemistry. This peer-reviewed journal has an impact factor of 3.837 as reported by the 2021 Journal Citat ...
. Linear and cyclic π systems bond to metals allowing organic complexes to bond to metals.
Linear systems
Ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
– π
In the most simple linear π systems, bonding to metals takes place by two interactions. Electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
is donated directly to the metal like a sigma bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of s ...
would be formed. Also, the metal can donate electron density back to the linear π system (ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
) from the metal’s d orbital to the empty π* orbital of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
.Miessler, G.A.; Tarr, D.A. Inorganic Chemistry. Pearson Education, Inc. 2010
Allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
groups can bond to metals as trihapto or monohapto ligands. Monohapto ligands bind mostly sigma orbitals and trihapto ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s bind using delocalized π orbitals. In essence the monohapto ligand binds the metal as an allyl group and the trihapto ligand binds over all three carbons, where the lowest energy π orbital donates electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
and the highest energy π orbital accepts electron density.
The allyl complex is diverse because it can alter the metal's electron count by transferring between a monohapto (1 electron, η1) and trihapto ligand (3 electrons, η3). This fluctuation allows stability when a two-electron-donating group bonds or breaks from the metal.
Cyclic systems
The specifics for binding of π cyclic systems are much more complex and depend on the electrons, theHOMO
''Homo'' () is the genus that emerged in the (otherwise extinct) genus ''Australopithecus'' that encompasses the extant species ''Homo sapiens'' ( modern humans), plus several extinct species classified as either ancestral to or closely relate ...
, and the LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
in each individual case of molecules. Cyclic π systems can bind monohapto or polyhapto depending on the individual situation. This means that π bonds
In chemistry, pi bonds (π bonds) are covalent bond, covalent chemical chemical bond, bonds, in each of which two lobes of an atomic orbital, orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap oc ...
can bind individually to the metal or there can be a single bond from the center of a benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
or cyclopentadienyl complex
A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups (, abbreviated as Cp−). Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (''η''5-) bonding mode. The metal–cyclopentadien ...
. Of course the bonding modes (η1, η3, η5, etc.) determine the number of donated electrons (1, 3, 5, etc.). The diversity of these cyclic complexes allows for a seemingly endless number of metallic structures.
Catalysis
The use of organometallic structures led by π–metal bonding plays an enormous role in the catalysis oforganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organ ...
s. The Stille reaction
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electro ...
is a widely known and important reaction in organic synthesis. π interactions with the Pd catalyst in this reaction are almost necessary in pushing this reaction to completion (alkyl groups transfer is rather slow).
Other widely known reactions based on π–metal catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
interactions are:
*Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a s ...
*Hiyama coupling
The Hiyama coupling is a palladium-catalyzed cross-coupling reaction of organosilanes with organic halides used in organic chemistry to form carbon–carbon bonds (C-C bonds). This reaction was discovered in 1988 by Tamejiro Hiyama and Yasuo Ha ...
*Kumada coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically n ...
*Negishi coupling
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) specie ...
*Petasis reaction
The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.
Reported in 1993 by Nicos Petasis ...
*Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vi ...
*Suzuki reaction
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, ...
.
π–metal interactions can also be involved directly with the function of ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s on the catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. Chemistry involving nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
catalysis of Suzuki reaction
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, ...
s was greatly affected by pyrazole
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
...
s and pyrazolates acting as coplanar ligand. The π interactions tied multiple pyrazoles and pyrazolates together around the nickel metal to cause reaction results.
Another π metal interaction directly involved with catalysis involves π stacking. Ferrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, a ...
is the standard example where the metal (iron) is trapped in between two cyclopentadienyl Cyclopentadienyl can refer to
*Cyclopentadienyl anion, or cyclopentadienide,
**Cyclopentadienyl ligand
*Cyclopentadienyl radical, •
*Cyclopentadienyl cation,
See also
*Pentadienyl
In organic chemistry, pentadienyl refers to the organic radic ...
ligands. These interactions are commonly referred to as sandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic deriv ...
s.
Specific research
Due to reasons explained earlier in the article, the bonding between a nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
and an electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
(II) leaves olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
susceptible to nucleophilic attack. This is true if the olefin is coordinated around Pd as the corner of a square planar
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corne ...
complex or as the side of a cationic 18-electron Pd complex. In both cases electron donating groups on the olefin stabilize the complex, but anionic electron donors actually destabilized the complex in the case of the 18-electron Pd complex. The authors of this research proposed that when the olefin π bond is aligned on the side of the square planar Pd complex, the π* backfilling of electron density from Pd to olefin is enhanced because the more electron withdrawing orbital of the π complex can overlap better with the electron donating orbital of Pd.
Anion–π interactions
Anion and π–aromatic systems (typically electron-deficient) create an interaction that is associated with the repulsive forces of the structures. These repulsive forces involve electrostatic and anion-induced polarized interactions. This force allows for the systems to be used as receptors and channels in supramolecular chemistry for applications in the medical (synthetic membranes, ion channels) and environmental fields (e.g. sensing, removal of ions from water). The first X-ray crystal structure that depicted anion–π interactions was reported in 2004. In addition to this being depicted in the solid state, there is also evidence that the interaction is present in solution.π-effects in biological systems
 π-effects have an important contribution to biological systems since they provide a significant amount of binding enthalpy. Neurotransmitters produce most of their biological effect by binding to the active site of a protein receptor. Pioneering work of Dennis A. Dougherty is a proof that such kind of binding stabilization is the effect of cation-π interactions of the
π-effects have an important contribution to biological systems since they provide a significant amount of binding enthalpy. Neurotransmitters produce most of their biological effect by binding to the active site of a protein receptor. Pioneering work of Dennis A. Dougherty is a proof that such kind of binding stabilization is the effect of cation-π interactions of the acetylcholine
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Part ...
(Ach) neurotransmitter. The structure of acetylcholine esterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acet ...
includes 14 highly conserved aromatic residues. The trimethyl ammonium group of Ach binds to the aromatic residue of tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
(Trp). The indole site provides a much more intense region of negative electrostatic potential than benzene and phenol residue of Phe and Tyr. S-Adenosyl methionine
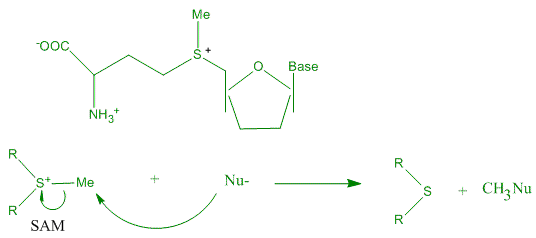
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throug ...
(SAM) can act as a catalyst for the transfer of methyl group from the sulfonium compound to nucleophile. The nucleophile can be any of a broad range structures including nucleic acids, proteins, sugars or C=C bond of lipids or steroids. The van der Waals contact between S-CH3 unit of SAM and the aromatic face of a Trp residue, in favorable alignment for catalysis assisted by cation-π interaction.
A great deal of circumstantial evidence places aromatic residues in the active site of a number of proteins that interact with cations but the presence of cation-π interaction in biological system does not rule out the conventional ion-pair interaction. In fact there is a good evidence for the existence of both type of interaction in model system.
In supramolecular assembly
 systems are important building blocks in
systems are important building blocks in supramolecular assembly
In chemistry, a supramolecular assembly is a complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules (e.g., a DNA double helix or an inclusion compound), or a defined num ...
because of their versatile noncovalent interactions with various functional groups. Particularly, recognition
Recognition may refer to:
*Award, something given in recognition of an achievement
Machine learning
*Pattern recognition, a branch of machine learning which encompasses the meanings below
Biometric
* Recognition of human individuals, or biomet ...
.
References
{{reflist, 2 Intermolecular forces