Phosphoramidite on:
[Wikipedia]
[Google]
[Amazon]
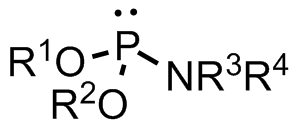 A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards
 A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s catalyzed by weak acids ''e.c''., triethylammonium chloride or 1''H''-tetrazole
A tetrazole is a chemical synthesis, synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound - a whitish crystalline powder with the f ...
. In these reactions, the incoming nucleophile replaces the NR2 moiety.
Applications
Nucleoside phosphoramidites
Phosphoramidites derived from protected nucleosides are referred to as nucleoside phosphoramidites and are widely used in chemical synthesis of DNA, RNA, and other nucleic acids and their analogs.As ligands
Certain phosphoramidites are also used as monodentate chiral ligands in asymmetric synthesis. A large group of such ligands is derived from the chiral diol BINOL and can be synthesised by reaction of BINOL with phosphorus trichloride to the chlorophosphite and then reaction with simple secondaryamine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s. This type of ligand was first used in 1996 in an asymmetric copper-catalysed addition of dialkylzincs to enones.
See also
* PhosphoramidateReferences
{{organophosphorus Phosphorus-nitrogen compounds Functional groups