Phosphinidene on:
[Wikipedia]
[Google]
[Amazon]
 Phosphinidenes (IUPAC: phosphanylidenes, formerly phosphinediyls) are low-valent phosphorus compounds analogous to carbenes and nitrenes, having the general structure RP. The parent phosphinidine has the formula PH. More common are the organic analogues where R = alkyl or aryl. In these compounds phosphorus has only 6 electrons in its valence level. Most phosphinidenes are highly reactive and short-lived, thereby complicating empirical studies on their chemical properties.
A variety of strategies have been employed to stabilize phosphinidenes (e.g. π-donation, Steric effects, steric protection, transition metal complexation), Furthermore reagents and systems have been developed that can generate and transfer phosphinidenes as intermediates in the synthesis of various organophosphorus compounds.
Phosphinidenes (IUPAC: phosphanylidenes, formerly phosphinediyls) are low-valent phosphorus compounds analogous to carbenes and nitrenes, having the general structure RP. The parent phosphinidine has the formula PH. More common are the organic analogues where R = alkyl or aryl. In these compounds phosphorus has only 6 electrons in its valence level. Most phosphinidenes are highly reactive and short-lived, thereby complicating empirical studies on their chemical properties.
A variety of strategies have been employed to stabilize phosphinidenes (e.g. π-donation, Steric effects, steric protection, transition metal complexation), Furthermore reagents and systems have been developed that can generate and transfer phosphinidenes as intermediates in the synthesis of various organophosphorus compounds.
 Like carbenes, phosphinidenes can exist in either a singlet state or triplet state, with the triplet state typically being more stable. The stability of these states and their relative energy difference (the singlet-triplet energy gap) depends on the substituents.
The ground state in the parent phosphinidene (PH) is a triplet that is 22 kcal/mol more stable than the lowest singlet state. This singlet-triplet energy gap is considerably larger than that of the simplest carbene Methylene (compound), methylene (9 kcal/mol).
Ab initio quantum chemistry methods, Ab initio calculations from Nguyen et al. found that alkyl- and silyl-substituted phosphinidenes have triplet ground states, possibly in-part due to a negative hyperconjugation. Substituents containing lone pairs (e.g. -NX2, -OX, -PX2 ,-SX) stabilize the singlet state, presumably by π-donation into an empty phosphorus 3p orbital; in most of these cases, the energies of the lowest singlet and triplet states were close to degenerate. A singlet ground state could be induced in amino- and phosphino-phosphinidenes by introducing bulky β-substituents, which are thought to destabilize the triplet state by distorting the pyramidal geometry through increased nuclear repulsion.
Like carbenes, phosphinidenes can exist in either a singlet state or triplet state, with the triplet state typically being more stable. The stability of these states and their relative energy difference (the singlet-triplet energy gap) depends on the substituents.
The ground state in the parent phosphinidene (PH) is a triplet that is 22 kcal/mol more stable than the lowest singlet state. This singlet-triplet energy gap is considerably larger than that of the simplest carbene Methylene (compound), methylene (9 kcal/mol).
Ab initio quantum chemistry methods, Ab initio calculations from Nguyen et al. found that alkyl- and silyl-substituted phosphinidenes have triplet ground states, possibly in-part due to a negative hyperconjugation. Substituents containing lone pairs (e.g. -NX2, -OX, -PX2 ,-SX) stabilize the singlet state, presumably by π-donation into an empty phosphorus 3p orbital; in most of these cases, the energies of the lowest singlet and triplet states were close to degenerate. A singlet ground state could be induced in amino- and phosphino-phosphinidenes by introducing bulky β-substituents, which are thought to destabilize the triplet state by distorting the pyramidal geometry through increased nuclear repulsion.
 Mass spectrometry, Molecular beam mass spectrometry has enabled the detection of the evolution of amino-phosphinidene fragments from a number of alkylamide derivatives (e.g. Me2NP+ and Me2NPH+ from Me2NPA) in the gas-phase at elevated temperatures.
Mass spectrometry, Molecular beam mass spectrometry has enabled the detection of the evolution of amino-phosphinidene fragments from a number of alkylamide derivatives (e.g. Me2NP+ and Me2NPH+ from Me2NPA) in the gas-phase at elevated temperatures.
 Density functional theory and Natural bond orbital, Natural bond orbital (NBO) calculations were used to gain insight into the structure and bonding of these phosphino-phosphinidenes. DFT calculations at the M06-2X/Def2-SVP level of theory on the phospino-phosphinidene with bulky 2,6-bis[4-tert-butylphenyl)methyl]-4-methylphenyl groups suggest that the tri-coordinated phosphorus atom exists in a planar environment. Calculations at the ''M06-2X/def2-TZVPP//M06-2X/def2-SVP'' level of theory were applied to a simplified model compound with diisopropylphenyl (Dipp) groups so as to reduce the computational cost for detailed NBO analysis. Inspection of the outputted wavefunctions shows that the HOMO and LUMO, HOMO and HOMO-1 are P-P π-bonding orbitals and the HOMO and LUMO, LUMO is a P-P π*-antibonding orbital. Further evidence of multiple bond character between the phosphorus atoms was provided by natural resonance theory and a large Kenneth B. Wiberg, Wiberg bond index (P1-P2: 2.34). Natural population analysis assigned a negative partial charge to the terminal phosphorus atom (-0.34 q) and a positive charge to the tri-coordinated phosphorus atom (1.16 q).
Density functional theory and Natural bond orbital, Natural bond orbital (NBO) calculations were used to gain insight into the structure and bonding of these phosphino-phosphinidenes. DFT calculations at the M06-2X/Def2-SVP level of theory on the phospino-phosphinidene with bulky 2,6-bis[4-tert-butylphenyl)methyl]-4-methylphenyl groups suggest that the tri-coordinated phosphorus atom exists in a planar environment. Calculations at the ''M06-2X/def2-TZVPP//M06-2X/def2-SVP'' level of theory were applied to a simplified model compound with diisopropylphenyl (Dipp) groups so as to reduce the computational cost for detailed NBO analysis. Inspection of the outputted wavefunctions shows that the HOMO and LUMO, HOMO and HOMO-1 are P-P π-bonding orbitals and the HOMO and LUMO, LUMO is a P-P π*-antibonding orbital. Further evidence of multiple bond character between the phosphorus atoms was provided by natural resonance theory and a large Kenneth B. Wiberg, Wiberg bond index (P1-P2: 2.34). Natural population analysis assigned a negative partial charge to the terminal phosphorus atom (-0.34 q) and a positive charge to the tri-coordinated phosphorus atom (1.16 q).
 Despite the negative charge on the terminal phosphorus atom, subsequent studies have shown that this particular phosphinidene is electrophilic at the phosphinidene center. This phosphino-phosphinidene reacts with a number of nucleophiles (CO, isocyanides, carbenes, phosphines, etc.) to form phosphinidene-nucleophile adducts Upon nucleophilic addition, the tri-coordinated phosphorus atom becomes non-planar, and it is postulated that the driving force of the reaction is provided by the instability of the phosphinidene's planar geometry.
Despite the negative charge on the terminal phosphorus atom, subsequent studies have shown that this particular phosphinidene is electrophilic at the phosphinidene center. This phosphino-phosphinidene reacts with a number of nucleophiles (CO, isocyanides, carbenes, phosphines, etc.) to form phosphinidene-nucleophile adducts Upon nucleophilic addition, the tri-coordinated phosphorus atom becomes non-planar, and it is postulated that the driving force of the reaction is provided by the instability of the phosphinidene's planar geometry.

 In 1989, Fritz et al. synthesized the phospha-Wittig species shown to the right. Phospha-Wittig compounds can be viewed as a phosphinidene stabilized by a phosphine. These compounds have been given the label of "phospha-Wittig" as they have two dominant resonance structures (a neutral form and a Zwitterion, zwitterionic form) that are analogous to those of the Ylide, phosphonium ylides that are used in the Wittig reaction.
Fritz et al. found that this particular phospha-Wittig reagent thermally decomposes at 20 °C to give tBu2PBr, LiBr, and cyclophosphanes. The authors proposed that the singlet phosphino-phosphinidene tBu2PP was formed as an intermediate in this reaction. Further evidence for this was provided by trapping experiments, where the thermal decomposition of the phospha-Wittig reagent in the presence of 3,4,-dimethyl-1,3-butadiene and cyclohexene gave rise to the products shown in the figure below.
In 1989, Fritz et al. synthesized the phospha-Wittig species shown to the right. Phospha-Wittig compounds can be viewed as a phosphinidene stabilized by a phosphine. These compounds have been given the label of "phospha-Wittig" as they have two dominant resonance structures (a neutral form and a Zwitterion, zwitterionic form) that are analogous to those of the Ylide, phosphonium ylides that are used in the Wittig reaction.
Fritz et al. found that this particular phospha-Wittig reagent thermally decomposes at 20 °C to give tBu2PBr, LiBr, and cyclophosphanes. The authors proposed that the singlet phosphino-phosphinidene tBu2PP was formed as an intermediate in this reaction. Further evidence for this was provided by trapping experiments, where the thermal decomposition of the phospha-Wittig reagent in the presence of 3,4,-dimethyl-1,3-butadiene and cyclohexene gave rise to the products shown in the figure below. 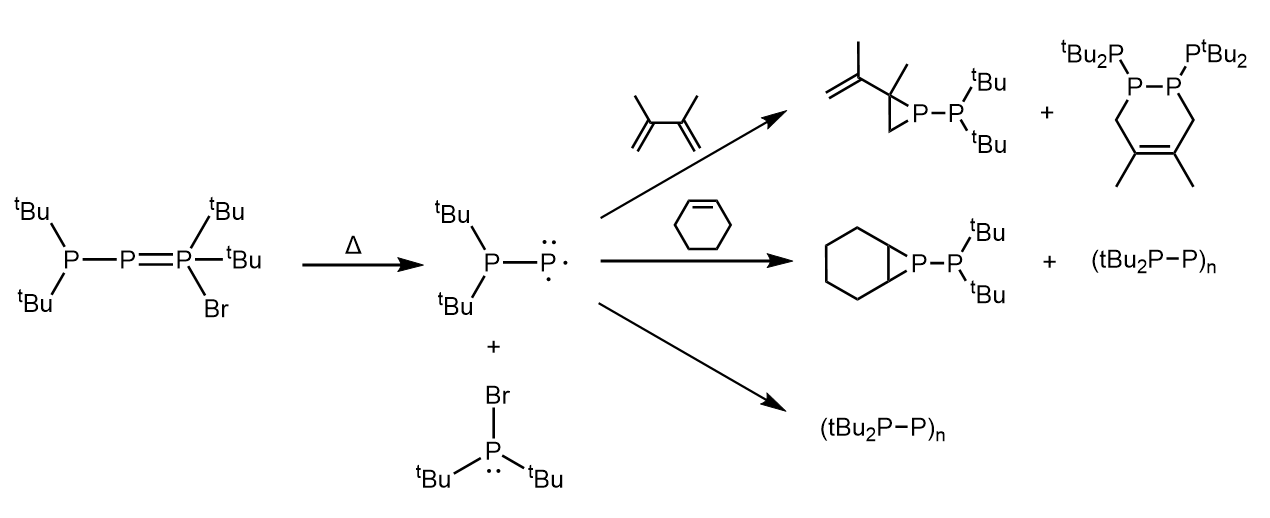
 Donor-stabilized terminal phosphinidene complexes are also known, which could release free phosphinidene complexes LnM=P-R at mild conditions by P-donor Dissociation (chemistry), dissociation reactions. The phosphinidene complexes decomposed to Allotropes of phosphorus, white phosphorus if no unsaturated substrates were provided.
Terminal phosphinidene complexes of the type Cp2M=P-R (M = Mo, W) can be obtained by combining aryl-dichlorophosphines RPCl2 with [Cp2MHLi]4.
Donor-stabilized terminal phosphinidene complexes are also known, which could release free phosphinidene complexes LnM=P-R at mild conditions by P-donor Dissociation (chemistry), dissociation reactions. The phosphinidene complexes decomposed to Allotropes of phosphorus, white phosphorus if no unsaturated substrates were provided.
Terminal phosphinidene complexes of the type Cp2M=P-R (M = Mo, W) can be obtained by combining aryl-dichlorophosphines RPCl2 with [Cp2MHLi]4.

 Phosphinidenes (IUPAC: phosphanylidenes, formerly phosphinediyls) are low-valent phosphorus compounds analogous to carbenes and nitrenes, having the general structure RP. The parent phosphinidine has the formula PH. More common are the organic analogues where R = alkyl or aryl. In these compounds phosphorus has only 6 electrons in its valence level. Most phosphinidenes are highly reactive and short-lived, thereby complicating empirical studies on their chemical properties.
A variety of strategies have been employed to stabilize phosphinidenes (e.g. π-donation, Steric effects, steric protection, transition metal complexation), Furthermore reagents and systems have been developed that can generate and transfer phosphinidenes as intermediates in the synthesis of various organophosphorus compounds.
Phosphinidenes (IUPAC: phosphanylidenes, formerly phosphinediyls) are low-valent phosphorus compounds analogous to carbenes and nitrenes, having the general structure RP. The parent phosphinidine has the formula PH. More common are the organic analogues where R = alkyl or aryl. In these compounds phosphorus has only 6 electrons in its valence level. Most phosphinidenes are highly reactive and short-lived, thereby complicating empirical studies on their chemical properties.
A variety of strategies have been employed to stabilize phosphinidenes (e.g. π-donation, Steric effects, steric protection, transition metal complexation), Furthermore reagents and systems have been developed that can generate and transfer phosphinidenes as intermediates in the synthesis of various organophosphorus compounds.
Electronic structure
 Like carbenes, phosphinidenes can exist in either a singlet state or triplet state, with the triplet state typically being more stable. The stability of these states and their relative energy difference (the singlet-triplet energy gap) depends on the substituents.
The ground state in the parent phosphinidene (PH) is a triplet that is 22 kcal/mol more stable than the lowest singlet state. This singlet-triplet energy gap is considerably larger than that of the simplest carbene Methylene (compound), methylene (9 kcal/mol).
Ab initio quantum chemistry methods, Ab initio calculations from Nguyen et al. found that alkyl- and silyl-substituted phosphinidenes have triplet ground states, possibly in-part due to a negative hyperconjugation. Substituents containing lone pairs (e.g. -NX2, -OX, -PX2 ,-SX) stabilize the singlet state, presumably by π-donation into an empty phosphorus 3p orbital; in most of these cases, the energies of the lowest singlet and triplet states were close to degenerate. A singlet ground state could be induced in amino- and phosphino-phosphinidenes by introducing bulky β-substituents, which are thought to destabilize the triplet state by distorting the pyramidal geometry through increased nuclear repulsion.
Like carbenes, phosphinidenes can exist in either a singlet state or triplet state, with the triplet state typically being more stable. The stability of these states and their relative energy difference (the singlet-triplet energy gap) depends on the substituents.
The ground state in the parent phosphinidene (PH) is a triplet that is 22 kcal/mol more stable than the lowest singlet state. This singlet-triplet energy gap is considerably larger than that of the simplest carbene Methylene (compound), methylene (9 kcal/mol).
Ab initio quantum chemistry methods, Ab initio calculations from Nguyen et al. found that alkyl- and silyl-substituted phosphinidenes have triplet ground states, possibly in-part due to a negative hyperconjugation. Substituents containing lone pairs (e.g. -NX2, -OX, -PX2 ,-SX) stabilize the singlet state, presumably by π-donation into an empty phosphorus 3p orbital; in most of these cases, the energies of the lowest singlet and triplet states were close to degenerate. A singlet ground state could be induced in amino- and phosphino-phosphinidenes by introducing bulky β-substituents, which are thought to destabilize the triplet state by distorting the pyramidal geometry through increased nuclear repulsion.
Case studies
Dibenzo-7-phosphanorbornadiene derivatives
One way to generate phosphinidines employs the decyclization of phosphaanthracene complexes. Treatment of a bulky phosphine chloride (RPCl2) with magnesium anthracene affords a dibenzo-7-phosphanorbornadiene compound (RPA). Under thermal conditions, the RPA compound (R = NiPr2) decomposes to yield anthracene; kinetic experiments found this decomposition to be first-order. It was hypothesized that the amino-phosphinidene iPr2NP is formed as a transient intermediate species, and this was corroborated by an experiment where Cyclohexa-1,3-diene, 1,3-cyclohexadiene was used as a trapping agent, forming ''anti''-iPr2NP(C6H8). Mass spectrometry, Molecular beam mass spectrometry has enabled the detection of the evolution of amino-phosphinidene fragments from a number of alkylamide derivatives (e.g. Me2NP+ and Me2NPH+ from Me2NPA) in the gas-phase at elevated temperatures.
Mass spectrometry, Molecular beam mass spectrometry has enabled the detection of the evolution of amino-phosphinidene fragments from a number of alkylamide derivatives (e.g. Me2NP+ and Me2NPH+ from Me2NPA) in the gas-phase at elevated temperatures.
Phosphino-phosphinidene
The first singlet phosphino-phosphinidene has been prepared using extremely bulky substituents. The authors prepared a chlorodiazaphospholidine with bulky (2,6-bis[(4-tert-butylphenyl)methyl]-4-methylphenyl) groups, and then synthesized the corresponding phosphaketene. Subsequent photolytic decarbonylation of the phosphaketene produced the phosphino-phosphinidene product as a yellow-orange solid that is stable at room temperature but decomposes immediately in the presence of air and moisture. 31P nuclear magnetic resonance spectroscopy, NMR spectroscopy shows assigned product peaks at 80.2 and -200.4 ppm, with a J-coupling constant of JPP = 883.7 Hz. The very high P-P coupling constant is indicative of P-P multiple bond character. The air/water sensitivity and high solubility of this compound prevented characterization by X-ray crystallography. Density functional theory and Natural bond orbital, Natural bond orbital (NBO) calculations were used to gain insight into the structure and bonding of these phosphino-phosphinidenes. DFT calculations at the M06-2X/Def2-SVP level of theory on the phospino-phosphinidene with bulky 2,6-bis[4-tert-butylphenyl)methyl]-4-methylphenyl groups suggest that the tri-coordinated phosphorus atom exists in a planar environment. Calculations at the ''M06-2X/def2-TZVPP//M06-2X/def2-SVP'' level of theory were applied to a simplified model compound with diisopropylphenyl (Dipp) groups so as to reduce the computational cost for detailed NBO analysis. Inspection of the outputted wavefunctions shows that the HOMO and LUMO, HOMO and HOMO-1 are P-P π-bonding orbitals and the HOMO and LUMO, LUMO is a P-P π*-antibonding orbital. Further evidence of multiple bond character between the phosphorus atoms was provided by natural resonance theory and a large Kenneth B. Wiberg, Wiberg bond index (P1-P2: 2.34). Natural population analysis assigned a negative partial charge to the terminal phosphorus atom (-0.34 q) and a positive charge to the tri-coordinated phosphorus atom (1.16 q).
Density functional theory and Natural bond orbital, Natural bond orbital (NBO) calculations were used to gain insight into the structure and bonding of these phosphino-phosphinidenes. DFT calculations at the M06-2X/Def2-SVP level of theory on the phospino-phosphinidene with bulky 2,6-bis[4-tert-butylphenyl)methyl]-4-methylphenyl groups suggest that the tri-coordinated phosphorus atom exists in a planar environment. Calculations at the ''M06-2X/def2-TZVPP//M06-2X/def2-SVP'' level of theory were applied to a simplified model compound with diisopropylphenyl (Dipp) groups so as to reduce the computational cost for detailed NBO analysis. Inspection of the outputted wavefunctions shows that the HOMO and LUMO, HOMO and HOMO-1 are P-P π-bonding orbitals and the HOMO and LUMO, LUMO is a P-P π*-antibonding orbital. Further evidence of multiple bond character between the phosphorus atoms was provided by natural resonance theory and a large Kenneth B. Wiberg, Wiberg bond index (P1-P2: 2.34). Natural population analysis assigned a negative partial charge to the terminal phosphorus atom (-0.34 q) and a positive charge to the tri-coordinated phosphorus atom (1.16 q).
 Despite the negative charge on the terminal phosphorus atom, subsequent studies have shown that this particular phosphinidene is electrophilic at the phosphinidene center. This phosphino-phosphinidene reacts with a number of nucleophiles (CO, isocyanides, carbenes, phosphines, etc.) to form phosphinidene-nucleophile adducts Upon nucleophilic addition, the tri-coordinated phosphorus atom becomes non-planar, and it is postulated that the driving force of the reaction is provided by the instability of the phosphinidene's planar geometry.
Despite the negative charge on the terminal phosphorus atom, subsequent studies have shown that this particular phosphinidene is electrophilic at the phosphinidene center. This phosphino-phosphinidene reacts with a number of nucleophiles (CO, isocyanides, carbenes, phosphines, etc.) to form phosphinidene-nucleophile adducts Upon nucleophilic addition, the tri-coordinated phosphorus atom becomes non-planar, and it is postulated that the driving force of the reaction is provided by the instability of the phosphinidene's planar geometry.

Phospha-Wittig fragmentation
 In 1989, Fritz et al. synthesized the phospha-Wittig species shown to the right. Phospha-Wittig compounds can be viewed as a phosphinidene stabilized by a phosphine. These compounds have been given the label of "phospha-Wittig" as they have two dominant resonance structures (a neutral form and a Zwitterion, zwitterionic form) that are analogous to those of the Ylide, phosphonium ylides that are used in the Wittig reaction.
Fritz et al. found that this particular phospha-Wittig reagent thermally decomposes at 20 °C to give tBu2PBr, LiBr, and cyclophosphanes. The authors proposed that the singlet phosphino-phosphinidene tBu2PP was formed as an intermediate in this reaction. Further evidence for this was provided by trapping experiments, where the thermal decomposition of the phospha-Wittig reagent in the presence of 3,4,-dimethyl-1,3-butadiene and cyclohexene gave rise to the products shown in the figure below.
In 1989, Fritz et al. synthesized the phospha-Wittig species shown to the right. Phospha-Wittig compounds can be viewed as a phosphinidene stabilized by a phosphine. These compounds have been given the label of "phospha-Wittig" as they have two dominant resonance structures (a neutral form and a Zwitterion, zwitterionic form) that are analogous to those of the Ylide, phosphonium ylides that are used in the Wittig reaction.
Fritz et al. found that this particular phospha-Wittig reagent thermally decomposes at 20 °C to give tBu2PBr, LiBr, and cyclophosphanes. The authors proposed that the singlet phosphino-phosphinidene tBu2PP was formed as an intermediate in this reaction. Further evidence for this was provided by trapping experiments, where the thermal decomposition of the phospha-Wittig reagent in the presence of 3,4,-dimethyl-1,3-butadiene and cyclohexene gave rise to the products shown in the figure below. 
Metal complexes
Terminal phosphinidine complexes
Terminal transition-metal-complexed phosphinidenes LnM=P-R are phosphorus analogs of transition metal carbene complexes. The first "metal-phosphinidine" was reported by Marinetti et al. They generated the transient species [(OC)5M=P-Ph] by fragmentation of 7-phosphanorbornadiene molybdenum and tungsten complexes inside a Mass spectrometry, mass spectrometer. Soon after, they discovered that these 7-phosphanorbornadiene complexes could be used to transfer the phosphinidene complex [(OC)5M=P-R] to various unsaturated substrates. Donor-stabilized terminal phosphinidene complexes are also known, which could release free phosphinidene complexes LnM=P-R at mild conditions by P-donor Dissociation (chemistry), dissociation reactions. The phosphinidene complexes decomposed to Allotropes of phosphorus, white phosphorus if no unsaturated substrates were provided.
Terminal phosphinidene complexes of the type Cp2M=P-R (M = Mo, W) can be obtained by combining aryl-dichlorophosphines RPCl2 with [Cp2MHLi]4.
Donor-stabilized terminal phosphinidene complexes are also known, which could release free phosphinidene complexes LnM=P-R at mild conditions by P-donor Dissociation (chemistry), dissociation reactions. The phosphinidene complexes decomposed to Allotropes of phosphorus, white phosphorus if no unsaturated substrates were provided.
Terminal phosphinidene complexes of the type Cp2M=P-R (M = Mo, W) can be obtained by combining aryl-dichlorophosphines RPCl2 with [Cp2MHLi]4.

Phosphinidine-based clusters
Metal clusters containing RP substituents are numerous. They typically arise by the reaction of metal carbonyls with primary phosphines (compounds with the formula RPH2). A partucularly well-studied case is , which forms from iron pentacarbonyl and phenylphosphine according to the following idealized equation: : A related example is the tert-butylphosphinidene complex (t-BuP)Fe3(CO)10.See also
* Carbene analog * *:Phosphorus compounds, Phosphorus compoundsReferences
{{Reflist Reactive intermediates Organophosphorus compounds Octet-deficient functional groups