Phenol Ether on:
[Wikipedia]
[Google]
[Amazon]
 In chemistry, a phenol ether (or aromatic ether) is an organic compound derived from
In chemistry, a phenol ether (or aromatic ether) is an organic compound derived from
 When substituents on aromatic rings are present, standard IUPAC nomenclature should be followed when naming aromatic compounds.
When substituents on aromatic rings are present, standard IUPAC nomenclature should be followed when naming aromatic compounds.
 However, this synthesis risks the self-condensation of alcohol itself (e.g. ethanol self-condenses to form diethyl ether). A more common and higher-yielding reaction is the
However, this synthesis risks the self-condensation of alcohol itself (e.g. ethanol self-condenses to form diethyl ether). A more common and higher-yielding reaction is the 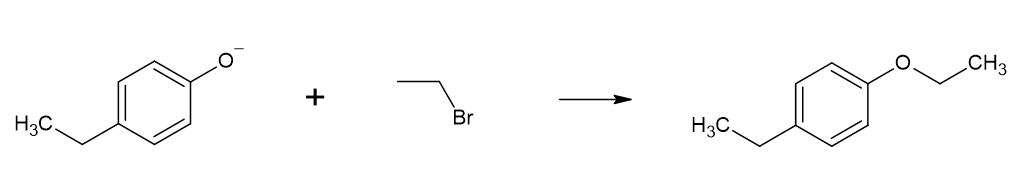 Bis-aryl ethers (such as diphenyl ether) cannot be synthesized through the Williamson ether synthesis, however, as aryl halides cannot undergo nucleophilic substitution. As such, an
Bis-aryl ethers (such as diphenyl ether) cannot be synthesized through the Williamson ether synthesis, however, as aryl halides cannot undergo nucleophilic substitution. As such, an 
 Phenol ethers are often utilized in pharmaceutical design as a substituent that acts as a hydrogen-bond acceptor but not a hydrogen-bond donor; this allows many oral medications to follow Lipinski’s rule of five. By replacing the acidic hydrogen on phenol with that of an alkyl group, the toxicity of phenols is also reduced; the LD50 of phenol in rats when administered orally is 317 mg/kg, compared to 3500-4000 mg/kg for anisole, the methyl ether. Furthermore, ethers are significantly more hydrophobic than phenols and can be more easily absorbed by the digestive system than the phenol substituent itself, and allows for oral intake of such medicines. For instance,
Phenol ethers are often utilized in pharmaceutical design as a substituent that acts as a hydrogen-bond acceptor but not a hydrogen-bond donor; this allows many oral medications to follow Lipinski’s rule of five. By replacing the acidic hydrogen on phenol with that of an alkyl group, the toxicity of phenols is also reduced; the LD50 of phenol in rats when administered orally is 317 mg/kg, compared to 3500-4000 mg/kg for anisole, the methyl ether. Furthermore, ethers are significantly more hydrophobic than phenols and can be more easily absorbed by the digestive system than the phenol substituent itself, and allows for oral intake of such medicines. For instance,
 In chemistry, a phenol ether (or aromatic ether) is an organic compound derived from
In chemistry, a phenol ether (or aromatic ether) is an organic compound derived from phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
(C6H5OH), where the hydroxyl (-OH) group is substituted with an alkoxy (-OR) group. Usually phenol ethers are synthesized through the condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
of phenol and an organic alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
; however, other known reactions regarding the synthesis of ethers
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ r ...
can be applied to phenol ethers as well. Anisole
Anisole, or methoxybenzene, is an organic compound with the formula . It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is mainly ...
(C6H5OCH3) is the simplest phenol ether, and is a versatile precursor for perfumes and pharmaceuticals. Vanillin
Vanillin is an organic compound with the molecular formula . It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the ethanolic extract of the vanilla bean. Synthetic vanillin ...
and ethylvanillin
Ethylvanillin is the organic compound with the chemical formula, formula (C2H5O)(HO)C6H3CHO. This colorless solid consists of a benzene ring with hydroxyl, ethoxy, and aldehyde, formyl groups on the 4, 3, and 1 positions, respectively. It is a Hom ...
are phenol ether derivatives commonly utilized in vanilla flavorings and fragrances, while diphenyl ether
Diphenyl ether is the organic compound with the formula ( C6 H5)2 O. It is a colorless, low-melting solid. This compound, the simplest diaryl ether, has a variety of niche applications.
Synthesis and reactions
Diphenyl ether was discovered by ...
is commonly used as a synthetic geranium fragrance. Phenol ethers are part of the chemical structure of a variety of medications, including quinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg ...
, an antimalarial drug, and dextromethorphan
Dextromethorphan, sold under the brand name Robitussin among others, is a cough suppressant used in many cough and Common cold, cold medicines. In 2022, the US Food and Drug Administration (FDA) approved the combination dextromethorphan/bupropi ...
, an over-the-counter cough suppressant.
Nomenclature
Phenol ethers follow the same nomenclature of regular ethers; ethers have the structure R-O-R’, but phenol ethers require that one of thesubstituents
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety (chemistry), moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that conta ...
to be a phenyl group (abbreviated Ph), signifying a simple general structure of Ph-O-R’. As a result, the IUPAC nomenclature
IUPAC nomenclature is a set of recommendations for naming chemical compounds and for describing chemistry and biochemistry in general. The International Union of Pure and Applied Chemistry (IUPAC) is the international authority on chemical nomenc ...
of phenol ethers will often take the form of “alkoxybenzene” or “phenoxyalkane,” where the alkane is some sort of hydrocarbon substituent.
The preference of the benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
ring in nomenclature relies on whether the alkane has more or less carbons than the benzene ring itself. Anisole is formally known as methoxybenzene, and is formed through the condensation of methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
(CH3OH) and phenol; due to the methyl group attached to the ethereal oxygen being smaller than the aromatic benzene ring, the benzene takes priority when naming the molecule. However, 1-phenoxyoctane has an octane substituent, which has a greater number of carbons than a benzene ring.
 When substituents on aromatic rings are present, standard IUPAC nomenclature should be followed when naming aromatic compounds.
When substituents on aromatic rings are present, standard IUPAC nomenclature should be followed when naming aromatic compounds.
Structure and properties
Phenol ethers, similarly to regular ethers, are lesshydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
than its precursors, phenols and alcohols, both of which can donate and accept hydrogen bonds. Phenol ethers, however, are still able to accept hydrogen bonds through the ethereal oxygen, allowing for its slight solubility in polar solvents. However, the presence of the aromatic ring reduces its solubility in polar solvents such as water and ethanol. Diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
has higher water solubility of 8 g per 100 mL, versus diphenyl ether, with a solubility of 0.002 g per 100 mL.
The presence of the aromatic ring also draws electrons away from the ethereal oxygen, making the hydrolysis of a phenol ether significantly more difficult than that of an alkyl ether. The ethereal oxygen must be significantly nucleophilic in order for the ether to undergo acid-catalyzed hydrolysis.
Preparation
Phenol ethers can be synthesized through an acid-catalyzed condensation of phenols and an alcohol.Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ar ...
include phenol itself, benzenediols, polyphenols, and other phenol-derived molecules.
 However, this synthesis risks the self-condensation of alcohol itself (e.g. ethanol self-condenses to form diethyl ether). A more common and higher-yielding reaction is the
However, this synthesis risks the self-condensation of alcohol itself (e.g. ethanol self-condenses to form diethyl ether). A more common and higher-yielding reaction is the Williamson ether synthesis
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ...
, where a phenol is converted by a strong base to the phenoxide
Phenolates (also called phenoxides) are anions, salts, and esters of phenols, containing the phenolate ion. They may be formed by reaction of phenols with strong base.
Properties
Alkali metal phenolates, such as sodium phenolate hydrolyze in aq ...
ion, which can subsequently be reacted with an alkyl halide via nucleophilic substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile) ...
to form the desired phenol ether. Primary alkyl halides work best, as secondary and tertiary alkyl halides prefer the E2 elimination
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 r ...
product. This ether synthesis removes the risk of self-condensation, and yields can be as high as 95% in the laboratory.
 Bis-aryl ethers (such as diphenyl ether) cannot be synthesized through the Williamson ether synthesis, however, as aryl halides cannot undergo nucleophilic substitution. As such, an
Bis-aryl ethers (such as diphenyl ether) cannot be synthesized through the Williamson ether synthesis, however, as aryl halides cannot undergo nucleophilic substitution. As such, an Ullmann condensation
The Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions.
Ullmann-type reaction ...
can be employed: an aryl halide is able to react with phenol (or its derivatives) to form a bis-aryl ether in the presence of a copper-based catalyst, such as copper(II) oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite, or so ...
.

Applications and occurrence
 Phenol ethers are often utilized in pharmaceutical design as a substituent that acts as a hydrogen-bond acceptor but not a hydrogen-bond donor; this allows many oral medications to follow Lipinski’s rule of five. By replacing the acidic hydrogen on phenol with that of an alkyl group, the toxicity of phenols is also reduced; the LD50 of phenol in rats when administered orally is 317 mg/kg, compared to 3500-4000 mg/kg for anisole, the methyl ether. Furthermore, ethers are significantly more hydrophobic than phenols and can be more easily absorbed by the digestive system than the phenol substituent itself, and allows for oral intake of such medicines. For instance,
Phenol ethers are often utilized in pharmaceutical design as a substituent that acts as a hydrogen-bond acceptor but not a hydrogen-bond donor; this allows many oral medications to follow Lipinski’s rule of five. By replacing the acidic hydrogen on phenol with that of an alkyl group, the toxicity of phenols is also reduced; the LD50 of phenol in rats when administered orally is 317 mg/kg, compared to 3500-4000 mg/kg for anisole, the methyl ether. Furthermore, ethers are significantly more hydrophobic than phenols and can be more easily absorbed by the digestive system than the phenol substituent itself, and allows for oral intake of such medicines. For instance, omeprazole
Omeprazole, sold under the brand names Prilosec and Losec, among others, is a medication used in the treatment of gastroesophageal reflux disease (GERD), peptic ulcer disease, and Zollinger–Ellison syndrome. It is also used to prevent up ...
, an oral medication that treats acid reflux, contains two phenol ether substituents.
Due to the increased hydrophobicity of phenol ethers compared to traditional phenols, phenol ethers are often present in the essential oils of plants. Anethole
Anethole (also known as anise camphor) is an organic compound that is widely used as a flavoring substance. It is a derivative of the aromatic compound allylbenzene and occurs widely in the essential oils of plants. It is in the class of phenylpr ...
, a simpler compound containing only one phenol ether substituent, is the main component in the oil of anise fruits. Elemicin
Elemicin is a phenylpropene, a natural organic compound, and is a constituent of several plant species' essential oils.
Natural occurrence
Elemicin is a constituent of the oleoresin and the essential oil of '' Canarium luzonicum'' (also referre ...
, a naturally-occurring organic compound containing three phenol ether substituents, is a major component in the oils of nutmeg
Nutmeg is the seed, or the ground spice derived from the seed, of several tree species of the genus '' Myristica''; fragrant nutmeg or true nutmeg ('' M. fragrans'') is a dark-leaved evergreen tree cultivated for two spices derived from its fru ...
and mace.{{pn, date=April 2021
References