Phase II Metabolism on:
[Wikipedia]
[Google]
[Amazon]
Drug metabolism is the metabolic breakdown of
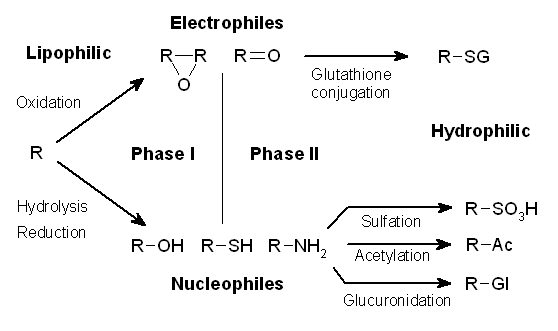 The metabolism of xenobiotics is often divided into three phases: modification, conjugation, and excretion. These reactions act in concert to detoxify xenobiotics and remove them from cells.
The metabolism of xenobiotics is often divided into three phases: modification, conjugation, and excretion. These reactions act in concert to detoxify xenobiotics and remove them from cells.
Drug metabolism database
*
Directory of P450-containing Systems
*
University of Minnesota Biocatalysis/Biodegradation Database
*
SPORCalc
* Drug metabolism *
*
* Microbial biodegradation *
* History ** {{Portal bar, Medicine Metabolism Hepatology Toxicology Pharmacokinetics Biodegradation
drug
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via insufflation (medicine), inhalation, drug i ...
s by living organism
An organism is any life, living thing that functions as an individual. Such a definition raises more problems than it solves, not least because the concept of an individual is also difficult. Many criteria, few of them widely accepted, have be ...
s, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell (biology), cell. The reactants, products, and Metabolic intermediate, intermediates of an enzymatic reaction are known as metabolites, which are ...
s that modify the chemical structure of xenobiotic
A xenobiotic is a chemical substance found within an organism that is not naturally produced or expected to be present within the organism. It can also cover substances that are present in much higher concentrations than are usual. Natural compo ...
s, which are compounds foreign to an organism's normal biochemistry, such as any drug
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via insufflation (medicine), inhalation, drug i ...
or poison
A poison is any chemical substance that is harmful or lethal to living organisms. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figurati ...
. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is the object of pharmacokinetics
Pharmacokinetics (from Ancient Greek ''pharmakon'' "drug" and ''kinetikos'' "moving, putting in motion"; see chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to describing how the body affects a specific su ...
. Metabolism is one of the stages (see ADME
ADME is the four-letter abbreviation (acronym) for absorption (pharmacokinetics), ''absorption'', distribution (pharmacology), ''distribution'', ''metabolism'', and ''excretion'', and is mainly used in fields such as pharmacokinetics and pharmacol ...
) of the drug's transit through the body that involves the breakdown of the drug so that it can be excreted by the body.
The metabolism of pharmaceutical drug
Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the ...
s is an important aspect of pharmacology
Pharmacology is the science of drugs and medications, including a substance's origin, composition, pharmacokinetics, pharmacodynamics, therapeutic use, and toxicology. More specifically, it is the study of the interactions that occur betwee ...
and medicine
Medicine is the science and Praxis (process), practice of caring for patients, managing the Medical diagnosis, diagnosis, prognosis, Preventive medicine, prevention, therapy, treatment, Palliative care, palliation of their injury or disease, ...
. For example, the rate of metabolism determines the duration and intensity of a drug's pharmacologic action. Drug metabolism also affects multidrug resistance
Multiple drug resistance (MDR), multidrug resistance or multiresistance is antimicrobial resistance shown by a species of microorganism to at least one antimicrobial drug in three or more antimicrobial categories. Antimicrobial categories are ...
in infectious disease
An infection is the invasion of tissue (biology), tissues by pathogens, their multiplication, and the reaction of host (biology), host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmis ...
s and in chemotherapy
Chemotherapy (often abbreviated chemo, sometimes CTX and CTx) is the type of cancer treatment that uses one or more anti-cancer drugs (list of chemotherapeutic agents, chemotherapeutic agents or alkylating agents) in a standard chemotherapy re ...
for cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
, and the actions of some drugs as substrates or inhibitors of enzymes involved in xenobiotic metabolism are a common reason for hazardous drug interaction In pharmaceutical sciences, drug interactions occur when a drug's mechanism of action is affected by the concomitant administration of substances such as foods, beverages, or other drugs. A popular example of drug–food interaction is the effect ...
s. These pathways are also important in environmental science, with the xenobiotic metabolism of microorganism
A microorganism, or microbe, is an organism of microscopic scale, microscopic size, which may exist in its unicellular organism, single-celled form or as a Colony (biology)#Microbial colonies, colony of cells. The possible existence of unseen ...
s determining whether a pollutant will be broken down during bioremediation
Bioremediation broadly refers to any process wherein a biological system (typically bacteria, microalgae, fungi in mycoremediation, and plants in phytoremediation), living or dead, is employed for removing environmental pollutants from air, wate ...
, or persist in the environment. The enzymes of xenobiotic metabolism, particularly the glutathione S-transferase
Glutathione ''S''-transferases (GSTs), previously known as ligandins, are a family of eukaryote, eukaryotic and prokaryote, prokaryotic Biotransformation#Phase II reaction, phase II metabolic isozymes best known for their ability to Catalysis, ...
s are also important in agriculture, since they may produce resistance to pesticide
Pesticides are substances that are used to control pests. They include herbicides, insecticides, nematicides, fungicides, and many others (see table). The most common of these are herbicides, which account for approximately 50% of all p ...
s and herbicide
Herbicides (, ), also commonly known as weed killers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page f ...
s.
Drug metabolism is divided into three phases. In phase I, enzymes such as cytochrome P450 oxidase
Cytochromes P450 (P450s or CYPs) are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in ''Escherich ...
s introduce reactive or polar groups into xenobiotics. These modified compounds are then conjugated to polar compounds in phase II reactions. These reactions are catalysed by transferase
In biochemistry, a transferase is any one of a class of enzymes that catalyse the transfer of specific functional groups (e.g. a methyl or glycosyl group) from one molecule (called the donor) to another (called the acceptor). They are involved ...
enzymes such as glutathione S-transferases. Finally, in phase III, the conjugated xenobiotics may be further processed, before being recognised by efflux transporters and pumped out of cells. Drug metabolism often converts lipophilic
Lipophilicity (from Greek language, Greek λίπος "fat" and :wikt:φίλος, φίλος "friendly") is the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such compounds are c ...
compounds into hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
products that are more readily excreted.
Permeability barriers and detoxification
The exact compounds an organism is exposed to will be largely unpredictable, and may differ widely over time; these are major characteristics of xenobiotic toxic stress. The major challenge faced by xenobiotic detoxification systems is that they must be able to remove the almost-limitless number of xenobiotic compounds from the complex mixture of chemicals involved in normalmetabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
. The solution that has evolved to address this problem is an elegant combination of physical barriers and low-specificity enzymatic systems.
All organisms use cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
s as hydrophobic permeability barriers to control access to their internal environment. Polar compounds cannot diffuse across these cell membranes, and the uptake of useful molecules is mediated through transport protein
A transport protein (variously referred to as a transmembrane pump, transporter, escort protein, acid transport protein, cation transport protein, or anion transport protein) is a protein that serves the function of moving other materials within ...
s that specifically select substrates from the extracellular mixture. This selective uptake means that most hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
molecules cannot enter cells, since they are not recognised by any specific transporters. In contrast, the diffusion of hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
compounds across these barriers cannot be controlled, and organisms, therefore, cannot exclude lipid
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing ...
-soluble xenobiotics using membrane barriers.
However, the existence of a permeability barrier means that organisms were able to evolve detoxification systems that exploit the hydrophobicity common to membrane-permeable xenobiotics. These systems therefore solve the specificity problem by possessing such broad substrate specificities that they metabolise almost any non-polar compound. Useful metabolites are excluded since they are polar, and in general contain one or more charged groups.
The detoxification of the reactive by-products of normal metabolism cannot be achieved by the systems outlined above, because these species are derived from normal cellular constituents and usually share their polar characteristics. However, since these compounds are few in number, specific enzymes can recognize and remove them. Examples of these specific detoxification systems are the glyoxalase system
The glyoxalase system is a set of enzymes that carry out the detoxification of methylglyoxal and the other reactive aldehydes that are produced as a normal part of metabolism. This system has been studied in both bacteria and eukaryotes. This detox ...
, which removes the reactive aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
methylglyoxal, and the various antioxidant systems that eliminate reactive oxygen species
In chemistry and biology, reactive oxygen species (ROS) are highly Reactivity (chemistry), reactive chemicals formed from diatomic oxygen (), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (H2O2), superoxide (O2−), hydroxyl ...
.
Phases of detoxification
 The metabolism of xenobiotics is often divided into three phases: modification, conjugation, and excretion. These reactions act in concert to detoxify xenobiotics and remove them from cells.
The metabolism of xenobiotics is often divided into three phases: modification, conjugation, and excretion. These reactions act in concert to detoxify xenobiotics and remove them from cells.
Phase I – modification
In phase I, a variety of enzymes act to introduce reactive and polar groups into their substrates. One of the most common modifications is hydroxylation catalysed by the cytochrome P-450-dependent mixed-function oxidase system. These enzyme complexes act to incorporate an atom of oxygen into nonactivated hydrocarbons, which can result in either the introduction of hydroxyl groups or N-, O- and S-dealkylation of substrates. The reaction mechanism of the P-450 oxidases proceeds through the reduction of cytochrome-bound oxygen and the generation of a highly-reactive oxyferryl species, according to the following scheme: :O2 + NADPH + H+ + RH → NADP+ + H2O + ROH Phase I reactions (also termed nonsynthetic reactions) may occur byoxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
, reduction, hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
, cyclization, decyclization, and addition of oxygen or removal of hydrogen, carried out by mixed function oxidases, often in the liver. These oxidative reactions typically involve a cytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for examp ...
monooxygenase (often abbreviated CYP), NADPH and oxygen. The classes of pharmaceutical drugs that utilize this method for their metabolism include phenothiazines, paracetamol
Paracetamol, or acetaminophen, is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely available over-the-counter drug sold under various brand names, including Tylenol and Panadol.
Parac ...
, and steroids. If the metabolites of phase I reactions are sufficiently polar, they may be readily excreted at this point. However, many phase I products are not eliminated rapidly and undergo a subsequent reaction in which an endogenous
Endogeny, in biology, refers to the property of originating or developing from within an organism, tissue, or cell.
For example, ''endogenous substances'', and ''endogenous processes'' are those that originate within a living system (e.g. an ...
substrate combines with the newly incorporated functional group to form a highly polar conjugate.
A common Phase I oxidation involves conversion of a C-H bond to a C-OH. This reaction sometimes converts a pharmacologically inactive compound (a prodrug
A prodrug is a pharmacologically inactive medication or compound that, after intake, is metabolized (i.e., converted within the body) into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be ...
) to a pharmacologically active one. By the same token, Phase I can turn a nontoxic molecule into a poisonous one ( toxification). Simple hydrolysis in the stomach is normally an innocuous reaction, however there are exceptions. For example, phase I metabolism converts acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
to HOCH2CN, which rapidly dissociates into formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
and hydrogen cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is ...
.
Phase I metabolism of drug candidates can be simulated in the laboratory using non-enzyme catalysts. This example of a biomimetic reaction tends to give products that often contains the Phase I metabolites. As an example, the major metabolite of the pharmaceutical trimebutine, desmethyltrimebutine (nor-trimebutine), can be efficiently produced by in vitro oxidation of the commercially available drug. Hydroxylation of an N-methyl group leads to expulsion of a molecule of formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
, while oxidation of the O-methyl groups takes place to a lesser extent.
Oxidation
* Cytochrome P450 monooxygenase system * Flavin-containing monooxygenase system *Alcohol dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to N ...
and aldehyde dehydrogenase
Aldehyde dehydrogenases () are a group of enzymes that catalyse the oxidation of aldehydes. They convert aldehydes (R–C(=O)) to carboxylic acids (R–C(=O)). The oxygen comes from a water molecule. To date, nineteen ALDH genes have ...
* Monoamine oxidase
Monoamine oxidases (MAO) () are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The fi ...
* Co-oxidation by peroxidase
Peroxidases or peroxide reductases ( EC numberbr>1.11.1.x are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides, and should not be confused with other ...
s
Reduction
* NADPH-cytochrome P450 reductase Cytochrome P450 reductase, also known as NADPH:ferrihemoprotein oxidoreductase, NADPH:hemoprotein oxidoreductase, NADPH:P450 oxidoreductase, P450 reductase, POR, CPR, CYPOR, is a membrane-bound enzyme required for electron transfer to cytochrome P450 in the microsome of the eukaryotic cell from a FAD- and FMN-containing enzyme NADPH:cytochrome P450 reductase The general scheme of electron flow in the POR/P450 system is: NADPH → FAD → FMN → P450 → O2 * Reduced (ferrous) cytochrome P450 During reduction reactions, a chemical can enter ''futile cycling'', in which it gains a free-radical electron, then promptly loses it tooxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
(to form a superoxide anion).
Hydrolysis
*Esterase
In biochemistry, an esterase is a class of enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis (and as such, it is a type of hydrolase).
A wide range of different esterases exist that differ ...
s and amidase
In enzymology, an amidase (, ''acylamidase'', ''acylase (misleading)'', ''amidohydrolase (ambiguous)'', ''deaminase (ambiguous)'', ''fatty acylamidase'', ''N-acetylaminohydrolase (ambiguous)'') is an enzyme that catalysis, catalyzes the hydrolysis ...
* Epoxide hydrolase
Phase II – conjugation
In subsequent phase II reactions, these activated xenobiotic metabolites are conjugated with charged species such asglutathione
Glutathione (GSH, ) is an organic compound with the chemical formula . It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources ...
(GSH), sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
, glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (G ...
, or glucuronic acid
Glucuronic acid (GCA, from ) is a uronic acid that was first isolated from urine (hence the name "uronic acid"). It is found in many natural gum, gums such as gum arabic ( 18%), xanthan, and kombucha tea and is important for the metabolism of ...
. Sites on drugs where conjugation reactions occur include carboxy (-COOH), hydroxy (-OH), amino
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
(NH2), and thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
(-SH) groups. Products of conjugation reactions have increased molecular weight and tend to be less active than their substrates, unlike Phase I reactions which often produce active metabolites. The addition of large anionic groups (such as GSH) detoxifies reactive electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
s and produces more polar metabolites that cannot diffuse across membranes, and may, therefore, be actively transported.
These reactions are catalysed by a large group of broad-specificity transferases, which in combination can metabolise almost any hydrophobic compound that contains nucleophilic or electrophilic groups. One of the most important classes of this group is that of the glutathione S-transferase
Glutathione ''S''-transferases (GSTs), previously known as ligandins, are a family of eukaryote, eukaryotic and prokaryote, prokaryotic Biotransformation#Phase II reaction, phase II metabolic isozymes best known for their ability to Catalysis, ...
s (GSTs).
Phase III – further modification and excretion
After phase II reactions, the xenobiotic conjugates may be further metabolized. A common example is the processing of glutathione conjugates to acetylcysteine (mercapturic acid) conjugates. Here, the γ-glutamate andglycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (G ...
residues in the glutathione molecule are removed by gamma-glutamyl transpeptidase
Gamma-glutamyltransferase (also γ-glutamyltransferase, GGT, gamma-GT, gamma-glutamyl transpeptidase; ) is a transferase (a type of enzyme) that catalyzes the transfer of gamma- glutamyl functional groups from molecules such as glutathion ...
and dipeptidases. In the final step, the cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
residue in the conjugate is acetylated.
Conjugates and their metabolites can be excreted from cells in phase III of their metabolism, with the anionic groups acting as affinity tags for a variety of membrane transporters of the multidrug resistance protein (MRP) family. These proteins are members of the family of ATP-binding cassette transporter
The ABC transporters, ATP synthase (ATP)-binding cassette transporters are a transport system superfamily that is one of the largest and possibly one of the oldest gene family, gene families. It is represented in all extant taxon, extant Phyl ...
s and can catalyse the ATP-dependent transport of a huge variety of hydrophobic anions, and thus act to remove phase II products to the extracellular medium, where they may be further metabolized or excreted.
Endogenous toxins
The detoxification of endogenous reactive metabolites such asperoxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...
s and reactive aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s often cannot be achieved by the system described above. This is the result of these species' being derived from normal cellular constituents and usually sharing their polar characteristics. However, since these compounds are few in number, it is possible for enzymatic systems to utilize specific molecular recognition to recognize and remove them. The similarity of these molecules to useful metabolites therefore means that different detoxification enzymes are usually required for the metabolism of each group of endogenous toxins. Examples of these specific detoxification systems are the glyoxalase system
The glyoxalase system is a set of enzymes that carry out the detoxification of methylglyoxal and the other reactive aldehydes that are produced as a normal part of metabolism. This system has been studied in both bacteria and eukaryotes. This detox ...
, which acts to dispose of the reactive aldehyde methylglyoxal
Methylglyoxal (MGO) is the organic compound with the formula CH3C(O)CHO. It is a reduced derivative of pyruvic acid. It is a reactive compound that is implicated in the biology of diabetes. Methylglyoxal is produced industrially by degradation ...
, and the various antioxidant
Antioxidants are Chemical compound, compounds that inhibit Redox, oxidation, a chemical reaction that can produce Radical (chemistry), free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants ...
systems that remove reactive oxygen species
In chemistry and biology, reactive oxygen species (ROS) are highly Reactivity (chemistry), reactive chemicals formed from diatomic oxygen (), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (H2O2), superoxide (O2−), hydroxyl ...
.
Sites
Quantitatively, the smooth endoplasmic reticulum of theliver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of var ...
cell is the principal organ of drug metabolism, although every biological tissue
In biology, tissue is an assembly of similar cells and their extracellular matrix from the same embryonic origin that together carry out a specific function. Tissues occupy a biological organizational level between cells and a complete or ...
has some ability to metabolize drugs.
Factors responsible for the liver's contribution to drug metabolism include that it is a large organ, that it is the first organ perfused by chemicals absorbed in the gut, and that there are very high concentrations of most drug-metabolizing enzyme systems relative to other organs.
If a drug is taken into the GI tract, where it enters hepatic circulation through the portal vein
The portal vein or hepatic portal vein (HPV) is a blood vessel that carries blood from the gastrointestinal tract, gallbladder, pancreas and spleen to the liver. This blood contains nutrients and toxins extracted from digested contents. Approxima ...
, it becomes well-metabolized and is said to show the ''first pass effect
The first pass effect (also known as first-pass metabolism or presystemic metabolism) is a phenomenon of drug metabolism at a specific location in the body which leads to a reduction in the concentration of the active drug before it reaches the ...
''.
Other sites of drug metabolism include epithelial cell
Epithelium or epithelial tissue is a thin, continuous, protective layer of Cell (biology), cells with little extracellular matrix. An example is the epidermis, the outermost layer of the skin. Epithelial (Mesothelium, mesothelial) tissues line ...
s of the gastrointestinal tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the Digestion, digestive system that leads from the mouth to the anus. The tract is the largest of the body's systems, after the cardiovascula ...
, lung
The lungs are the primary Organ (biology), organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the Vertebral column, backbone on either side of the heart. Their ...
s, kidney
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organ (anatomy), organs that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation. They are located on the left and rig ...
s, and the skin
Skin is the layer of usually soft, flexible outer tissue covering the body of a vertebrate animal, with three main functions: protection, regulation, and sensation.
Other animal coverings, such as the arthropod exoskeleton, have different ...
.
These sites are usually responsible for localized toxicity reactions.
Factors affecting drug metabolism
The duration and intensity of pharmacological action of most lipophilic drugs are determined by the rate they are metabolized to inactive products. The Cytochrome P450 monooxygenase system is a crucial pathway in this regard. In general, anything that ''increases'' the rate of metabolism (e.g., enzyme induction) of a pharmacologically active metabolite will ''decrease'' the duration and intensity of the drug action. The opposite is also true, as inenzyme inhibition
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its Enzyme activity, activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which Substrate (biochemistry), substrate molecules are converted ...
. However, in cases where an enzyme is responsible for metabolizing a pro-drug into a drug, enzyme induction can accelerate this conversion and increase drug levels, potentially causing toxicity.
Various ''physiological'' and ''pathological'' factors can also affect drug metabolism. Physiological factors that can influence drug metabolism include age, individual variation (e.g., pharmacogenetics), enterohepatic circulation
Enterohepatic circulation is the circulation of biliary acids, bilirubin, drugs or other substances from the liver to the bile, followed by entry into the small intestine, absorption by the enterocyte and transport back to the liver. Enterohepa ...
, nutrition
Nutrition is the biochemistry, biochemical and physiology, physiological process by which an organism uses food and water to support its life. The intake of these substances provides organisms with nutrients (divided into Macronutrient, macro- ...
, sex differences or gut microbiota
Gut microbiota, gut microbiome, or gut flora are the microorganisms, including bacteria, archaea, fungi, and viruses, that live in the digestive tracts of animals. The gastrointestinal metagenome is the aggregate of all the genomes of the g ...
. This last factor has significance because gut microorganisms are able to chemically modify the structure of drugs through degradation and biotransformation processes, thus altering the activity and toxicity of drugs. These processes can decrease the efficacy of drugs, as is the case of digoxin
Digoxin (better known as digitalis), sold under the brand name Lanoxin among others, is a medication used to treat various heart disease, heart conditions. Most frequently it is used for atrial fibrillation, atrial flutter, and heart failure. ...
in the presence of ''Eggerthella lenta'' in the microbiota. Genetic variation ( polymorphism) accounts for some of the variability in the effect of drugs.
In general, drugs are metabolized more slowly in fetal
A fetus or foetus (; : fetuses, foetuses, rarely feti or foeti) is the unborn offspring of a viviparous animal that develops from an embryo. Following the embryonic stage, the fetal stage of development takes place. Prenatal development is a ...
, neonatal
In common terminology, a baby is the very young offspring of adult human beings, while infant (from the Latin word ''infans'', meaning 'baby' or 'child') is a formal or specialised synonym. The terms may also be used to refer to Juvenile (orga ...
and elderly
Old age is the range of ages for people nearing and surpassing life expectancy. People who are of old age are also referred to as: old people, elderly, elders, senior citizens, seniors or older adults. Old age is not a definite biological sta ...
human
Humans (''Homo sapiens'') or modern humans are the most common and widespread species of primate, and the last surviving species of the genus ''Homo''. They are Hominidae, great apes characterized by their Prehistory of nakedness and clothing ...
s and animal
Animals are multicellular, eukaryotic organisms in the Biology, biological Kingdom (biology), kingdom Animalia (). With few exceptions, animals heterotroph, consume organic material, Cellular respiration#Aerobic respiration, breathe oxygen, ...
s than in adult
An adult is an animal that has reached full growth. The biological definition of the word means an animal reaching sexual maturity and thus capable of reproduction. In the human context, the term ''adult'' has meanings associated with social an ...
s. Inherited genetic variations in drug metabolising enzymes result in their different catalytic activity levels. For example, N-acetyltransferases (involved in ''Phase II'' reactions), individual variation creates a group of people who acetylate slowly (''slow acetylators'') and those who acetylate quickly (''rapid acetylators''), split roughly 50:50 in the population of Canada. However, variability in ''NAT2'' alleles distribution across different populations is high and some ethnicities have higher proportion of slow acetylators. This variation in metabolising capacity may have dramatic consequences, as the N-acetyltransferase#Importance_in_Humans, slow acetylators are more prone to dose-dependent toxicity. NAT2 enzyme is a primary metaboliser of antituberculosis (isoniazid), some antihypertensive (hydralazine), anti-arrythmic drugs (procainamide), antidepressants (phenelzine) and many more and increased toxicity as well as drug adverse reactions in slow acetylators have been widely reported. Similar phenomenons of altered metabolism due to inherited variations have been described for other drug-metabolising enzymes, like CYP2D6, CYP3A4, Dihydropyrimidine dehydrogenase (NADP+), DPYD, UDP glucuronosyltransferase 1 family, polypeptide A1, UGT1A1. ''DPYD'' and ''UGT1A1'' genotyping is now required before administration of the corresponding substrate compounds (Fluorouracil, 5-FU and capecitabine for DPYD and irinotecan for UGT1A1) to determine the activity of DPYD and UGT1A1 enzyme and reduce the dose of the drug in order to avoid severe adverse reactions.
Dose, frequency, route of administration, tissue distribution and protein binding of the drug affect its metabolism. ''Pathological factors'' can also influence drug metabolism, including liver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of var ...
, kidney
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organ (anatomy), organs that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation. They are located on the left and rig ...
, or heart diseases.
''In silico'' modelling and simulation methods allow drug metabolism to be predicted in virtual patient populations prior to performing clinical studies in human subjects. This can be used to identify individuals most at risk from adverse reaction.
History
Studies on how people transform the substances that they ingest began in the mid-nineteenth century, with chemists discovering that organic chemicals such as benzaldehyde could be oxidized and conjugated to amino acids in the human body. During the remainder of the nineteenth century, several other basic detoxification reactions were discovered, such as methylation, acetylation, and sulfonation. In the early twentieth century, work moved on to the investigation of the enzymes and pathways that were responsible for the production of these metabolites. This field became defined as a separate area of study with the publication by Richard Tecwyn Williams, Richard Williams of the book ''Detoxication mechanisms'' in 1947. This modern biochemical research resulted in the identification of glutathione ''S''-transferases in 1961, followed by the discovery of cytochrome P450s in 1962, and the realization of their central role in xenobiotic metabolism in 1963.See also
* Biodegradation * Microbial biodegradationReferences
Further reading
* * * * *External links
* Databases *Drug metabolism database
*
Directory of P450-containing Systems
*
University of Minnesota Biocatalysis/Biodegradation Database
*
SPORCalc
* Drug metabolism *
*
* Microbial biodegradation *
* History ** {{Portal bar, Medicine Metabolism Hepatology Toxicology Pharmacokinetics Biodegradation