Petrenko-Kritschenko Piperidone Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Petrenko-Kritschenko reaction is a classic multicomponent-
 In contrast to the Robinson synthesis, it does not employ dialdehydes like
In contrast to the Robinson synthesis, it does not employ dialdehydes like

name reaction
A name reaction (or named reaction) is a chemical reaction named after its discoverer(s) or developer(s). Among the tens of thousands of organic reactions that are known, hundreds of such reactions are typically identified by the eponym. Well-know ...
Jie-Jack Li; "Name reactions in heterocyclic chemistry"; 2005 John Wiley & Sons; ; pp313 that is closely related to the Robinson–Schöpf tropinone
Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. I ...
synthesis, but was published 12 years earlier.
Classic reaction
In the original publicationP. Petrenko-Kritschenko "Über die Kondensation des Acetondicarbonsäureesters mit Aldehyden, Ammoniak und Aminen" Journal für Praktische Chemie Volume 85, Issue 1, pages 1–37, 20 May 1912; diethyl-α-ketoglurate, a derivative ofacetonedicarboxylic acid
Acetonedicarboxylic acid, 3-oxoglutaric acid or β-ketoglutaric acid is a simple dicarboxylic acid with the formula . β-Ketoglutarate does not have the biological activity exhibited by α-ketoglutarate.
Preparation
Acetonedicarboxylic acid ca ...
, is used in combination with ammonia and benzaldehyde. The relative stereochemistry was not elucidated in the original publication, structural analysis using X-rays or NMR was not available in these days. In the absence of ammonia or ammonium salts a 4-oxotetrahydropyran is formed.P. Petrenko-Kritschenko "Über Tetrahydropyronverbindungen" Journal für Praktische Chemie; Volume 60, Issue 1, pages 140–158, 27 December 1899;
 In contrast to the Robinson synthesis, it does not employ dialdehydes like
In contrast to the Robinson synthesis, it does not employ dialdehydes like succinaldehyde
Succinaldehyde or Butanedial is an organic compound with the formula . It is a colorless viscous liquid. Typical of some other saturated dialdehydes, succinaldehyde is handled as the hydrates or methanol-derived acetal. It is a precursor to tropi ...
or glutaraldehyde
Glutaraldehyde is an organic compound with the formula . The molecule consists of a five carbon chain doubly terminated with formyl (CHO) groups. It is usually used as a solution in water, and such solutions exists as a collection of hydrates, ...
but simpler aldehydes like benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-li ...
. Therefore, the product of the reaction is not a bicyclic structure (see tropinone
Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commodity during World War I. Tropinone and the alkaloids cocaine and atropine all share the same tropane core structure. I ...
and pseudopelletierine) but a 4-piperidone. The synthesis of tropinone can be seen as a variation of the Petrenko-Kritschenko reaction in which the two aldehyde functions are covalently linked in a single molecule. Apart from the Hantzsch synthesis the Petrenko-Kritschenko reaction is one of the few examples in which a symmetric pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
precursor can be obtained in a multicomponent ring-condensation reaction followed by an oxidation. The oxidation by chromium trioxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula . It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.
This compound is a dark-purple solid ...
in acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
leads to a symmetrically substituted 4-pyridone, decarboxylation yields the 3,5-unsubstituted derivative.
Modern variants
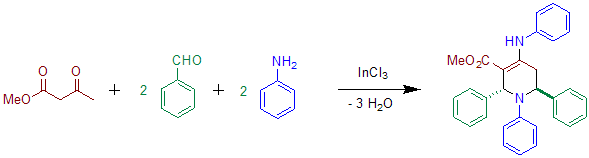
Acetoacetate can be used instead of diethyl-α-ketoglurate in the presence ofindium
Indium is a chemical element; it has Symbol (chemistry), symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are la ...
salts.Clarke, Paul A.; Zaytzev, Andrey V.; Whitwood, Adrian C. "Pot, atom and step economic (PASE) synthesis of highly functionalized piperidines: a five-component condensation" Tetrahedron Letters Volume 48, Issue 30, 23 July 2007, Pages 5209–5212; The use of aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
has also been reported in the original Publication. The product of this reaction shows transoid configuration of the phenyl groups at C-2 and C-6.

Natural product synthesis
The reaction has been used to prepare precoccinellin, an alkaloid found in certainladybugs
Coccinellidae () is a widespread family of small beetles. They are commonly known as ladybugs in North America and ladybirds in the United Kingdom; "lady" refers to mother Mary. Entomologists use the names ladybird beetles or lady beetles ...
.

Applications to coordination chemistry
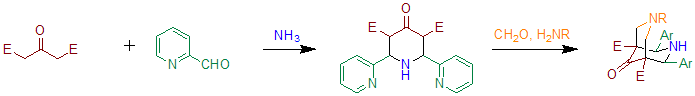
When benzaldehyde is substituted with 2-pyridinecarboxaldehyde the reaction can be used to prepare precursors for bispidone-ligands.Comba, Peter; Kerscher, Marion; Merz, Michael; Müller, Vera; Pritzkow, Hans; Remenyi, Rainer; Schiek, Wolfgang; Xiong, Yun "Structural Variation in Transition-Metal Bispidine Compounds" Chemistry – A European Journal Volume 8, Issue 24, pages 5750–5760, 16 December 2002; Essentially this method is based on two subsequent Petrenko-Kritschenko reactions. These ligands can be used to prepare compounds containinghigh-valent iron
High-valent iron commonly denotes compounds and intermediates in which iron is found in a formal oxidation state > +3 that show a number of bonds > 6 with a coordination number ≤ 6. The ferrate(VI) ion eO4sup>2− was the first structure in t ...
, that are able to oxidize cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
in the presence of hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
.

References
{{ReflistExternal links
* A picture of Paul Petrenko-Kritschenko taken at the Kazan School of Chemistry in 1928 (1st row, first on the left): http://www.ksu.ru/chmku/images/30b.jpg Name reactions Ring forming reactions