PCl5 on:
[Wikipedia]
[Google]
[Amazon]
Phosphorus pentachloride is the
 In solutions of polar solvents, undergoes self-
In solutions of polar solvents, undergoes self-
 It also converts
It also converts
The period 3 chlorides
{{Authority control Phosphorus chlorides Hypervalent molecules Phosphorus(V) compounds
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the formula . It is one of the most important phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
chlorides/oxychlorides, others being and . finds use as a chlorinating reagent. It is a colourless, water-sensitive solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
, although commercial samples can be yellowish and contaminated with hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
.
Structure
The structures for the phosphorus chlorides are invariably consistent withVSEPR theory
Valence shell electron pair repulsion (VSEPR) theory ( , ) is a conceptual model, model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gill ...
. The structure of depends on its environment. Gaseous and molten is a neutral molecule with trigonal bipyramidal
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identi ...
geometry and (''D''3h) symmetry
Symmetry () in everyday life refers to a sense of harmonious and beautiful proportion and balance. In mathematics, the term has a more precise definition and is usually used to refer to an object that is Invariant (mathematics), invariant und ...
. The hypervalent
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded Octet rule, octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. P ...
nature of this species (as well as of , see below) can be explained with the inclusion of non-bonding molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
s (molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century. The MOT explains the paramagnetic nature of O2, whic ...
) or resonance
Resonance is a phenomenon that occurs when an object or system is subjected to an external force or vibration whose frequency matches a resonant frequency (or resonance frequency) of the system, defined as a frequency that generates a maximu ...
(valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
). This trigonal bipyramidal structure persists in nonpolar solvents, such as and . In the solid state is an ionic compound
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions (Cation, cations) and negatively charged ions (Anion, anions), which results in a compound with no net electric charge (electrica ...
called tetrachlorophosphonium hexachlorophosphate formulated .
 In solutions of polar solvents, undergoes self-
In solutions of polar solvents, undergoes self-ionization
Ionization or ionisation is the process by which an atom or a molecule acquires a negative or positive Electric charge, charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged at ...
. Dilute solutions dissociate according to the following equilibrium:
:
At higher concentrations, a second equilibrium becomes more prevalent:
:
The cation and the anion are tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
and octahedral
In geometry, an octahedron (: octahedra or octahedrons) is any polyhedron with eight faces. One special case is the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. Many types of i ...
, respectively. At one time, in solution was thought to form a dimeric structure, , but this suggestion is not supported by Raman spectroscopic measurements.
Related pentachlorides
and also adopt trigonal bipyramidal structures. The relevant bond distances are 211 pm (As−Cleq), 221 pm (As−Clax), 227 pm (Sb−Cleq), and 233.3 pm (Sb−Clax). At low temperatures, converts to the dimer, dioctahedral , structurally related toniobium pentachloride
Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid. It hydrolyzes in air, and samples are often contaminated with small amounts of NbOCl3. It is often used as a precursor to other compounds of niobium. NbCl ...
.
Preparation
is prepared by the chlorination of . This reaction is used to produce around 10,000 tonnes of per year (as of 2000). : (Δ''H'' = −124 kJ/mol) exists in equilibrium with andchlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
, and at 180 °C the degree of dissociation is about 40%. Because of this equilibrium, samples of often contain chlorine, which imparts a greenish coloration.
Reactions
Hydrolysis
In its most characteristic reaction,reacts
''React'' (from Spanish: ''Reacciona'') is a book by Rosa María Artal published in Spain in 2011 by Aguilar, which compiles articles by José Luis Sampedro, Baltasar Garzón, Federico Mayor Zaragoza, Javier Pérez de Albéniz, Javier López Faca ...
upon contact with water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
to release hydrogen chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd ...
and give phosphorus oxides. The first hydrolysis product is phosphorus oxychloride
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phos ...
:
:
In hot water, hydrolysis proceeds completely to orthophosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, w ...
:
:
Lewis acidity
Phosphorus pentachloride is a Lewis acid. This property underpins many of its characteristic reactions, autoionization, chlorinations, hydrolysis. A well studied adduct is .Chlorination of organic compounds
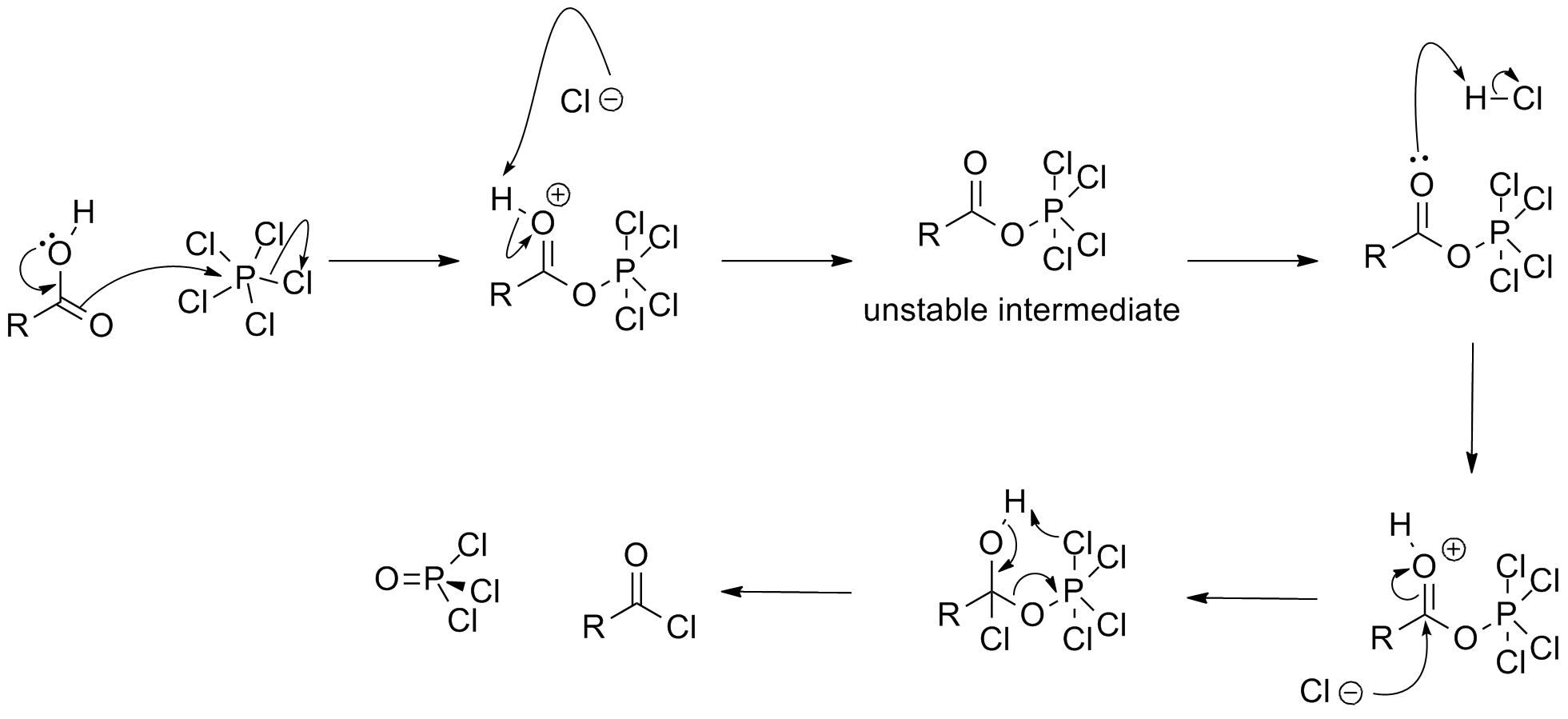
In synthetic chemistry, two classes of chlorination are usually of interest: oxidative chlorinations and substitutive chlorinations. Oxidative chlorinations entail the transfer of from the reagent to the substrate. Substitutive chlorinations entail replacement of O or OH groups with chloride. can be used for both processes. Upon treatment with ,carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s convert to the corresponding acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
. The following mechanism has been proposed:
: It also converts
It also converts alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s to alkyl chlorides. Thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately Volatility (chemistry), volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a Halogenation, chlorinating reagen ...
is more commonly used in the laboratory because the resultant sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
is more easily separated from the organic products than is .
reacts with a tertiary amides, such as dimethylformamide
Dimethylformamide, DMF is an organic compound with the chemical formula . Its structure is . Commonly abbreviated as DMF (although this initialism is sometimes used for 2,5-dimethylfuran, dimethylfuran, or dimethyl fumarate), this colourless liqui ...
(DMF), to give dimethylchloromethyleneammonium chloride, which is called the Vilsmeier reagent
The Vilsmeier reagent is an organic compound with the formula CH3)2NCHCll. It is a salt consisting of the N,N-dimethyliminium cation ( CH3)2N=CHClsup>+) and chloride anion. Depending on the particular reaction, the anion can vary. In typica ...
, . More typically, a related salt is generated from the reaction of DMF and . Such reagents are useful in the preparation of derivatives of benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-li ...
by formylation and for the conversion of C−OH groups into C−Cl groups.
It is especially renowned for the conversion of C=O groups to groups. For example, benzophenone
Benzophenone is a naturally occurring organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone has been found in some fungi, fruits and plants, including grapes. It is a white solid with a low melting point and ros ...
and phosphorus pentachloride react to give the diphenyldichloromethane
Diphenyldichloromethane is an organic compound with the formula (C6H5)2CCl2. It is a colorless solid that is used as a precursor to other organic compounds.
Synthesis
It is prepared from carbon tetrachloride and anhydrous aluminium chloride as c ...
:
:
The electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
character of is highlighted by its reaction with styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
to give, after hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
, phosphonic acid derivatives.
Comparison with related reagents
Both and convert groups to the chloride . The pentachloride is however a source of chlorine in many reactions. It chlorinates allylic andbenzylic
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group ().
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substituent, ...
CH bonds. bears a greater resemblance to , also a source of . For oxidative chlorinations on the laboratory scale, sulfuryl chloride is often preferred over since the gaseous by-product is readily separated.
Chlorination of inorganic compounds
As for the reactions with organic compounds, the use of has been superseded by . The reaction ofphosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula Phosphorus, P4Oxygen, O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desic ...
and produces :
:
chlorinates nitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, is an intermediate in the s ...
to form unstable nitryl chloride
Nitryl chloride is a volatile inorganic compound with formula ClNO2. At standard conditions it is a gas.
Formation
Nitryl chloride can be formed in the reaction of dinitrogen pentoxide with chlorides or hydrogen chloride:
:N2O5 + 2HCl → 2ClNO2 ...
:
:
:
is a precursor for lithium hexafluorophosphate
Lithium hexafluorophosphate is an inorganic compound with the formula Li PF6. It is a white crystalline powder.
Production
LiPF6 is manufactured by reacting phosphorus pentachloride with hydrogen fluoride and lithium fluoride
:PCl5 + LiF + 5 H ...
, . Lithium hexafluorophosphate is a commonly employed salt in electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
s in lithium ion batteries
A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. Li-ion batteries are characterized by higher specific energy, energy d ...
. is produced by the reaction of with lithium fluoride
Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid that transitions to white with decreasing crystal size.
Its structure is analogous to that of sodium chloride, but it is much less soluble in water. ...
, with lithium chloride
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorid ...
as a side product:
:
Safety
is a dangerous chemical as it reacts violently with water. It is also corrosive when in contact with skin. It is toxic and can be fatal when inhaled.History
Phosphorus pentachloride was first prepared in 1808 by the English chemistHumphry Davy
Sir Humphry Davy, 1st Baronet (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several Chemical element, e ...
. Davy's analysis of phosphorus pentachloride was inaccurate; the first accurate analysis was provided in 1816 by the French chemist Pierre Louis Dulong
Pierre Louis Dulong FRS FRSE (; ; 12 February 1785 – 19 July 1838) was a French physicist and chemist. He is remembered today largely for the law of Dulong and Petit, although he was much-lauded by his contemporaries for his studies into ...
. On p. 148, Dulong presented the correct analysis of phosphorus pentachloride (which is 14.9% phosphorus and 85.1% chlorine by weight, vs. Dulong's values of 15.4% and 84.6%, respectively).
See also
*Phosphorus halides
In chemistry, there are three series of binary phosphorus halides, containing phosphorus in the oxidation states +5, +3 and +2. All compounds have been described, in varying degrees of detail, although serious doubts have been cast on the existenc ...
* Phosphorus trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic ...
* Phosphoryl chloride
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosp ...
* Phosphorus trifluorodichloride
Phosphorus trifluorodichloride is a chemical compound with the chemical formula . It is a toxic colorless gas with a disagreeable odor, and it turns into a liquid at −8 °C. The covalent molecule trigonal bipyramidal molecular geometry. The ...
References
External links
The period 3 chlorides
{{Authority control Phosphorus chlorides Hypervalent molecules Phosphorus(V) compounds