Oxidative Coupling Of Phenols on:
[Wikipedia]
[Google]
[Amazon]
Oxidative coupling of phenols is a
 Oxidative phenol couplings can occur through either inner sphere or outer sphere processes. In inner sphere processes, the phenolic substrate coordinates to the metal center to give a phenoxide complex. Oxidation to the phenoxide occurs via electron transfer or hydrogen atom abstraction. The resulting reactive intermediate can engage in downstream chemical processes which can occur via either coordinated (inner-sphere) or non-coordinated coupling partners.
Oxidative phenol couplings can occur through either inner sphere or outer sphere processes. In inner sphere processes, the phenolic substrate coordinates to the metal center to give a phenoxide complex. Oxidation to the phenoxide occurs via electron transfer or hydrogen atom abstraction. The resulting reactive intermediate can engage in downstream chemical processes which can occur via either coordinated (inner-sphere) or non-coordinated coupling partners.
 Radical-radical reactions are simple to envision but unlikely since it requires the coexistence of two long-lived radicals. Instead, the phenol or phenoxy radical adds to another phenol or phenoxide. The initial C-C bond forming process is followed hydrogen atom abstraction and tautomerization.
Couplings where metal catalysts are not involved generally proceed via the radical-phenol mechanism.
Although select examples of unsymmetrical homocouplings are known, they are notoriously challenging to design and are often arrived at empirically.
Enantioselective asymmetric phenol oxidative couplings are not well-established or general yet, however there exist reports leveraging asymmetric vanadium catalysts to enantioselectively homocouple phenols. In contrast, much progress has been made in asymmetric 2-napthol couplings using Ru, Cu, V, and Fe catalysts, which have had a large impact on the development of
Radical-radical reactions are simple to envision but unlikely since it requires the coexistence of two long-lived radicals. Instead, the phenol or phenoxy radical adds to another phenol or phenoxide. The initial C-C bond forming process is followed hydrogen atom abstraction and tautomerization.
Couplings where metal catalysts are not involved generally proceed via the radical-phenol mechanism.
Although select examples of unsymmetrical homocouplings are known, they are notoriously challenging to design and are often arrived at empirically.
Enantioselective asymmetric phenol oxidative couplings are not well-established or general yet, however there exist reports leveraging asymmetric vanadium catalysts to enantioselectively homocouple phenols. In contrast, much progress has been made in asymmetric 2-napthol couplings using Ru, Cu, V, and Fe catalysts, which have had a large impact on the development of
 The first example of an oxidative
The first example of an oxidative 
 Laccases often effect oxidative couplings, sometimes forming C-O linkages.
Selective C–O coupling of phenols are represented by few examples in synthetic chemistry. In many cases, selective C–O coupling can only be achieved if all ortho and para-positions on the arene are blocked. Poor C–O coupling selectivity is likely due to the lack of radical spin-density on oxygen after phenol oxidation, resulting in kinetic trapping of C–C coupling products.
Laccases often effect oxidative couplings, sometimes forming C-O linkages.
Selective C–O coupling of phenols are represented by few examples in synthetic chemistry. In many cases, selective C–O coupling can only be achieved if all ortho and para-positions on the arene are blocked. Poor C–O coupling selectivity is likely due to the lack of radical spin-density on oxygen after phenol oxidation, resulting in kinetic trapping of C–C coupling products.
chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
wherein two phenolic compound
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are c ...
s are coupled via an oxidative process. Oxidative phenol couplings are often catalyzed by transition metal complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or ...
es including V, Cr, Mn, Cu, Fe, among others. Such reactions often form C–C, or C–O bonds between the coupling partners and can be employed as either homo- or cross-couplings.
Mechanism
A representative example is the reaction ofphenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
with a solution of vanadium tetrachloride
Vanadium tetrachloride is the inorganic compound with the formula V Cl4. This reddish-brown liquid serves as a useful reagent for the preparation of other vanadium compounds.
Synthesis, bonding, basic properties
With one more valence electron ...
, which yields about 60% yield of three isomeric dihydroxybiphenyl compounds. The isomer ratio and yields are unaffected by the reagent/substrate ratio. Vanadium tetrachloride is known to effect one-electron oxidations, which is invoked in this conversion.
 Radical-radical reactions are simple to envision but unlikely since it requires the coexistence of two long-lived radicals. Instead, the phenol or phenoxy radical adds to another phenol or phenoxide. The initial C-C bond forming process is followed hydrogen atom abstraction and tautomerization.
Couplings where metal catalysts are not involved generally proceed via the radical-phenol mechanism.
Although select examples of unsymmetrical homocouplings are known, they are notoriously challenging to design and are often arrived at empirically.
Enantioselective asymmetric phenol oxidative couplings are not well-established or general yet, however there exist reports leveraging asymmetric vanadium catalysts to enantioselectively homocouple phenols. In contrast, much progress has been made in asymmetric 2-napthol couplings using Ru, Cu, V, and Fe catalysts, which have had a large impact on the development of
Radical-radical reactions are simple to envision but unlikely since it requires the coexistence of two long-lived radicals. Instead, the phenol or phenoxy radical adds to another phenol or phenoxide. The initial C-C bond forming process is followed hydrogen atom abstraction and tautomerization.
Couplings where metal catalysts are not involved generally proceed via the radical-phenol mechanism.
Although select examples of unsymmetrical homocouplings are known, they are notoriously challenging to design and are often arrived at empirically.
Enantioselective asymmetric phenol oxidative couplings are not well-established or general yet, however there exist reports leveraging asymmetric vanadium catalysts to enantioselectively homocouple phenols. In contrast, much progress has been made in asymmetric 2-napthol couplings using Ru, Cu, V, and Fe catalysts, which have had a large impact on the development of BINAP
BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This Optical isomerism, chiral diphosphines, diphosphine ligand is widely used in chiral synthesis, asymmetric synthesis. It consists of a pair of 2-diphe ...
-type ligands used asymmetric catalysis.
Scope
Lignin
Lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidit ...
, a polyphenol
Polyphenols () are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include phenolic acids, flavonoids, tannic acid, and ellagitannin, some of which have been used historically as ...
that is found in most plants, is a very abundant form of biomass that arises, in part, by oxidative coupling of phenols. Lignins are particularly important in the formation of cell wall
A cell wall is a structural layer that surrounds some Cell type, cell types, found immediately outside the cell membrane. It can be tough, flexible, and sometimes rigid. Primarily, it provides the cell with structural support, shape, protection, ...
s, especially in wood
Wood is a structural tissue/material found as xylem in the stems and roots of trees and other woody plants. It is an organic materiala natural composite of cellulosic fibers that are strong in tension and embedded in a matrix of lignin t ...
and bark
Bark may refer to:
Common meanings
* Bark (botany), an outer layer of a woody plant such as a tree or stick
* Bark (sound), a vocalization of some animals (which is commonly the dog)
Arts and entertainment
* ''Bark'' (Jefferson Airplane album), ...
, because they lend rigidity and do not rot easily. Chemically, lignins are polymers made by cross-linking phenolic precursors.
Organic synthesis
 The first example of an oxidative
The first example of an oxidative phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
coupling in synthetic chemistry can be traced to Julius Löwe’s 1868 synthesis of ellagic acid, accomplished by heating gallic acid with arsenic acid.
In the synthesis of complex organic compounds, oxidative phenol couplings are sometimes employed. The reaction is attractive for their atom economy
Atom economy (atom efficiency/percentage) is the conversion efficiency of a chemical process in terms of all atoms involved and the desired products produced. The simplest definition was introduced by Barry Trost in 1991 and is equal to the rati ...
because it avoid pre-functionalized starting materials often required in traditional redox-neutral cross-couplings. Oxidative phenol couplings, however, often suffer from over-oxidation, especially since the intended coupled product is more oxidizable (has a lower oxidation potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respe ...
) than the starting material. In such cases, the catalyst can be quenched or poisoned by engaging in off-cycle redox processes with the product. Additionally, the product may oxidize further, giving way to higher-order oligomers
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
.
Selectivity issues may arise during oxidative phenol couplings between C–C coupled and C–O coupled products. Moreover, stereoselectivity is an important consideration if the resulting biphenol compound displays axial chirality
In chemistry, axial chirality is a special case of chirality (chemistry), chirality in which a molecule contains two pairs of chemical groups in a non-planar arrangement about an axis of chirality so that the molecule is not superposable on its mi ...
or atropoisomerism. Selectivity between homo- and hetero-coupled products must be considered, and can often be addressed through transition-metal catalysis.

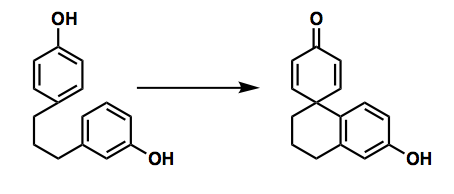
Intramolecular phenol couplings
Intramolecular oxidative phenol couplings have long been known. The most well-studied examples of such transformations are those yielding spirocyclic phenol-dienone coupled products. The coupling partners in an intramolecular coupling must approach in a near-parallel arrangement to allow for orbital overlap; these stringent geometric restraints on pre-cyclized compounds often render the process sluggish, if possible.
C–O couplings
:Nonphenolic arene couplings
Oxidative couplings have also been studied between phenols and nonphenolic compounds including anilines, beta-ketoesters/malonates/malononitriles, electron-rich arenes, olefins, and other functional groups.References
{{reflist Coupling reactions Biphenyls Organic oxidation reactions