Organotellurium Compound on:
[Wikipedia]
[Google]
[Amazon]
Organotellurium chemistry describes the synthesis and properties of organotellurium compounds,
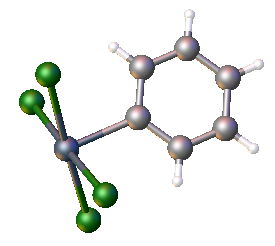 One departure from sulfur and selenium chemistry is the availability of the tetrachloride, TeCl4. It reacts with arenes to give aryltellurium trichlorides:
:ArH + TeCl4 → ArTeCl3 + HCl
For electron-rich arenes, the disubstitution occurs
:ArH + ArTeCl3 → Ar2TeCl2 + HCl
Tellurium tetrachloride adds across alkenes and alkynes to the chloro tellurium trichlorides:
:RCH=CH2 + TeCl4 → RCH(Cl)-CH2TeCl3
Organotellurium trichlorides adopt dimeric structures, reflecting the
One departure from sulfur and selenium chemistry is the availability of the tetrachloride, TeCl4. It reacts with arenes to give aryltellurium trichlorides:
:ArH + TeCl4 → ArTeCl3 + HCl
For electron-rich arenes, the disubstitution occurs
:ArH + ArTeCl3 → Ar2TeCl2 + HCl
Tellurium tetrachloride adds across alkenes and alkynes to the chloro tellurium trichlorides:
:RCH=CH2 + TeCl4 → RCH(Cl)-CH2TeCl3
Organotellurium trichlorides adopt dimeric structures, reflecting the
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s containing a carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
-tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fou ...
chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
. Organotellurium chemistry is a lightly studied area, in part because of it having few applications.
Functional groups
The tellurium analogues of commonorganosulfur
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur der ...
and organoselenium functional groups are known. Tellurol
Tellurols are analogues of alcohol (chemistry), alcohols and phenols where tellurium replaces oxygen. Tellurols, selenols, and thiols have similar properties, but tellurols are the least stable. Although they are fundamental representatives of orga ...
s are however unstable with respect to oxidation to the ditellurides. Commonly encountered organotellurium compounds are diorganomono- and ditellurides, R2Te and (RTe)2, respectively. Two other families of organotellurium(IV) compounds are well developed: R4−xTeClx and the telluroxides (R2TeO).
Synthesis and reactions
Reduced organotellurium compounds
Reduced organotellurium compounds are commonly obtained from NaHTe and lithium telluride: :Li2Te + 2 RBr → R2Te + 2 LiBr A direct route to organolithium compounds starts from reactions of organolithium orGrignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
s and Te:
:Te + ArLi → ArTeLi
Butyl lithium gives the telluride similarly:
:Te + BuLi → BuTeLi
Organotelluride anions can be oxidized or alkylated:
:2 RTeLi + 0.5 O2 + H2O → RTeTeR + 2 LiOH
:RTeLi + R'Br → RTeR' + LiBr
Diorganoditellurides are valued intermediates, especially the aryl derivatives such as diphenyl ditelluride:
:Ar2Te2 + RLi → RTeAr + LiTeR
:Ph2Te2 + 2 Li → 2 LiTePh
Derivatives of TeCl4
 One departure from sulfur and selenium chemistry is the availability of the tetrachloride, TeCl4. It reacts with arenes to give aryltellurium trichlorides:
:ArH + TeCl4 → ArTeCl3 + HCl
For electron-rich arenes, the disubstitution occurs
:ArH + ArTeCl3 → Ar2TeCl2 + HCl
Tellurium tetrachloride adds across alkenes and alkynes to the chloro tellurium trichlorides:
:RCH=CH2 + TeCl4 → RCH(Cl)-CH2TeCl3
Organotellurium trichlorides adopt dimeric structures, reflecting the
One departure from sulfur and selenium chemistry is the availability of the tetrachloride, TeCl4. It reacts with arenes to give aryltellurium trichlorides:
:ArH + TeCl4 → ArTeCl3 + HCl
For electron-rich arenes, the disubstitution occurs
:ArH + ArTeCl3 → Ar2TeCl2 + HCl
Tellurium tetrachloride adds across alkenes and alkynes to the chloro tellurium trichlorides:
:RCH=CH2 + TeCl4 → RCH(Cl)-CH2TeCl3
Organotellurium trichlorides adopt dimeric structures, reflecting the Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
ity of the Te(IV) center. The dimers are cleaved by halides and other Lewis bases:
:RTeCl3 + Cl− → RTeCl4−
The anions RTeCl4− (and the related adducts RTeCl3L) adopt square pyramidal structures with the electronegative groups in the plane.
Organotellurium(IV) chlorides are susceptible to substitution reactions where by chloride is replaced by other halides and pseudohalides. The TeClx group can also be removed with Raney nickel
Raney nickel , also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable s ...
.
Organotellurium(IV) compounds participate in Stille reactions:
:
Telluroxides
Telluroxides are generally related tosulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s and selenoxides in terms of their structures. Unlike their lighter analogues however, they polymerize (reversibly) when crystallized. Analogous to selenoxide oxidation, allylic telluroxides undergo ,3 sigmatropic rearrangements forming allylic alcohols after hydrolysis. Also analogous to the selenoxide elimination, certain telluroxides give alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s upon heating.
Te(VI)
Hexamethylpertellurane was prepared by oxidation of tetramethyltellurium withxenon difluoride
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as th ...
. The resulting TeF2(CH3)4 is then treated with dimethylzinc
Dimethylzinc, also known as zinc methyl, DMZ, or DMZn, is a toxic organozinc compound with the chemical formula . It belongs to the large series of similar compounds such as diethylzinc.
Preparation
It is formed by the action of methyl iodide on ...
:
:
:
The octahedral compounds TeAr6 have also been prepared.
Applications
Dimethyl telluride
Dimethyl telluride is an organotelluride compound, formula ( CH3)2 Te, also known by the abbreviation DMTe.
This was the first material used to grow epitaxial cadmium telluride and mercury cadmium telluride using metalorganic vapour phase epit ...
is used to in metalorganic vapour phase epitaxy
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. ...
where it serves as a volatile source of Te. It is the only organotellurium compound that has been quantified in environmental samples.
Telluroethers undergo a variant of the selenoxide elimination.
References
{{Authority control