Organorhodium Compound on:
[Wikipedia]
[Google]
[Amazon]
 Organorhodium chemistry is the
Organorhodium chemistry is the
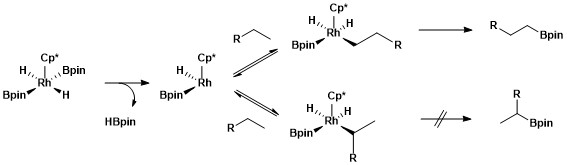
 The catalytically active species is the
The catalytically active species is the
\overset + + H2 -> ce\text] \overset

 Organorhodium chemistry is the
Organorhodium chemistry is the chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
of organometallic compounds
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, an ...
containing a rhodium
Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isot ...
-carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
, and the study of rhodium and rhodium compounds as catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
s in organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
s.
Stable organorhodium compounds and transient organorhodium intermediates are used as catalyst such as in olefin hydroformylation
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes () from alkenes (). This chemical reaction entails the net addition of a formyl group () and a hydrogen ...
, olefin hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
, olefin isomerization and the Monsanto process
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Che ...
.
Classification based on principal oxidation states
Organometallic rhodium compounds share many characteristics with those of iridium, but less so with cobalt. Rhodium can exist inoxidation states
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms are fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Concep ...
of −3 to +5, but rhodium(I) and rhodium(III) are the more common. Rhodium(I) compounds (d8 configuration) usually occur with square planar or trigonal bipyramidal geometries, while rhodium (III) compounds (d6 configuration) typically have an octahedral geometry.
Rhodium(0)
Rhodium(0) complexes are binary carbonyls, the principal examples beingtetrarhodium dodecacarbonyl
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12. This dark-red crystalline solid is the smallest binary rhodium carbonyl that can be handled as a solid under ambient conditions. It is used as a catalyst in organic ...
, Rh4(CO)12, and hexadecacarbonylhexarhodium
Hexadecacarbonylhexarhodium is a metal carbonyl cluster with the formula Rh6(CO)16. It exists as purple-brown crystals that are slightly soluble in dichloromethane and chloroform. It is the principal binary carbonyl of rhodium.
Discovery and s ...
, Rh6(CO)16. These compounds are obtained by reductive carbonylation
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemis ...
of rhodium(III) salts or Rh2Cl2(CO)4. In contrast to the stability of the homologous Co2(CO)8, Rh2(CO)8 is very labile.
Rhodium(I)
Rhodium(I) complexes are importanthomogeneous catalyst
In chemistry, homogeneous catalysis is catalysis where the catalyst is in same phase as reactants, principally by a soluble catalyst in a solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in di ...
s. Common complexes include bis(triphenylphosphine)rhodium carbonyl chloride, chlorobis(ethylene)rhodium dimer, cyclooctadiene rhodium chloride dimer, chlorobis(cyclooctene)rhodium dimer, dicarbonyl(acetylacetonato)rhodium(I)
Dicarbonyl(acetylacetonato)rhodium(I) is an organorhodium compound with the formula Rh(O2C5H7)(CO)2. The compound consists of two CO ligands and an acetylacetonate. It is a dark green solid that dissolves in acetone and benzene, giving yellow s ...
, and rhodium carbonyl chloride
Rhodium carbonyl chloride is an organorhodium compound with the formula Rh2Cl2(CO)4. It is a red-brown volatile solid that is soluble in nonpolar organic solvents. It is a precursor to other rhodium carbonyl complexes, some of which are useful ...
. Although not formally organometallic, Wilkinson's catalyst
Wilkinson's catalyst (chloridotris(triphenylphosphine)rhodium(I)) is a coordination complex of rhodium with the formula hCl(PPh3)3 where 'Ph' denotes a phenyl group. It is a red-brown colored solid that is soluble in hydrocarbon solv ...
(RhCl(PPh3)3), is included in the list of important catalysts. The simple olefin complexes chlorobis(ethylene)rhodium dimer, chlorobis(cyclooctene)rhodium dimer, and cyclooctadiene rhodium chloride dimer are often used as sources of "RhCl", exploiting the lability of the alkene ligands or their susceptibility to removal by hydrogenation. (η5- Cp)RhL2 are derived from Rh2Cl2L4 (L = CO, C2H4).
Rhodium(II)
Unlike the prevalence of cobalt(II) complexes, compounds of rhodium(II) are rare. Thesandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by hapticity, haptic, covalent bonds to two arene compound, arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ...
rhodocene
Rhodocene is a chemical compound with the formula . Each molecule contains an atom of rhodium bound between two planar aromaticity, aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich compound, sandwich arrangement ...
is one example, even it exists in equilibrium with a dimeric Rh(I) derivative. Although not organometallic, rhodium(II) acetate
Rhodium(II) acetate is the coordination compound with the formula Rh2(AcO)4, where AcO− is the acetate ion (). This dark green powder is slightly soluble in polar solvents, including water. It is used as a catalyst for cyclopropanation of alke ...
(Rh2(OAc)4) catalyzes cyclopropanation
In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane () Ring (chemistry), rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroid insectic ...
s via organometallic intermediates. Rhodium(II) porphyrin
Porphyrins ( ) are heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (). In vertebrates, an essential member of the porphyrin group is heme, w ...
complexes react with methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
.
Rhodium(III)
Rhodium is usually supplied commercially in the Rh(III) oxidation state, the main starting reagent being hydratedrhodium trichloride
Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isoto ...
. The latter reacts with olefins and with CO to give organometallic complexes, often concomitant with reduction to Rh(I). Cyclopentadienyl complexes of rhodium include the half-sandwich compound
Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples in ...
pentamethylcyclopentadienyl rhodium dichloride dimer
Pentamethylcyclopentadienyl rhodium dichloride dimer is an organometallic compound with the formula C5(CH3)5RhCl2)sub>2, commonly abbreviated p*RhCl2sub>2 This dark red air-stable diamagnetic solid is a reagent in organometallic chemistry.
Stru ...
.
Rhodium(V)
Strong donor ligands - hydride, silyl, boryl - are required to stabilize Rh(V). This oxidation state is invoked inborylation
Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydroge ...
reactions.

Metallacycles
Cyclometalatedrhodium
Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isot ...
compounds constitute an important class of organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
chemistry. Although such compounds are well documented in the literature rhodium(III) cyclometalates with azo function are spare. A typical example of this category viz. novel hexacoordinated orthometalated rhodium(III) thiolato complex trans- h(C∧N∧S)Cl(PPh3)2was synthesized from benzyl 2-(phenylazo)phenyl thioether and RhCl3·3H2O in the presence of excess PPh3 via in situ C(sp2)−H and C(sp3)−S bond scissions. This is the first example for a coordination compound of (phenylazo)thiolate ligand. The mechanism of formation of orthometalated azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a azo compound, N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide c ...
derivative was described to proceed via initial coordination of azo-nitrogen followed by electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
substitution at the pendant phenyl ring. PPh3 plays a crucial role in the C(sp3)−S cleavage process. Reductive cleavage by single electron transfer
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spont ...
(SET) mechanism is likely to be operative for the C−S bond cleavage. Unlike analogous (phenylazo)phenolato compound the orthometalated thiolato complex exhibits a fully reversible oxidative wave at 0.82 V vs Ag/AgCl and this response is supposed to be primarily centered on the thiolato sulfur atom.
Main applications
Despite its high cost, rhodium is heavily relied on as a commercial catalyst.Acetic acid and acetic anhydride syntheses
TheMonsanto process
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Che ...
is an industrial method for the making of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
by catalytic carbonylation
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemis ...
of methanol, although it has largely been supplanted by the iridium-based Cativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an ...
.
anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
''cis''- h(CO)2I2sup>−. which undergoes oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
with methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one h ...
. The related Tennessee Eastman acetic anhydride process affords acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of c ...
by carbonylation
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemis ...
of methyl acetate
Methyl acetate, also known as MeOAc, acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is a flammable liquid with a characteristically pleasant smell reminiscent of some glues and nail polish remo ...
.
: CH3CO2CH3 + CO → (CH3CO)2O
Hydroformylation
Hydroformylation
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes () from alkenes (). This chemical reaction entails the net addition of a formyl group () and a hydrogen ...
s often rely on rhodium-based catalysts. Water-soluble catalysts have also been developed. They facilitate the separation of the products from the catalyst.
:Hydrogenation
Wilkinson's catalyst is used as ahomogeneous catalyst
In chemistry, homogeneous catalysis is catalysis where the catalyst is in same phase as reactants, principally by a soluble catalyst in a solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in di ...
for the hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
of olefins. The mechanism of catalysis involves oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
of H2, π-complexation of alkene, migratory insertion
In organometallic chemistry, a migratory insertion is a type of chemical reaction, reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiate ...
(intramolecular hydride transfer or olefin insertion), and reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
.
Cationic organorhodium(I) catalysts are useful for asymmetric hydrogenation
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from oth ...
s, which are applied to bioactive products such as pharmaceutical agents
Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the m ...
and agrochemicals
An agrochemical or agrichemical, a contraction of ''agricultural chemical'', is a chemical product used in industrial agriculture. Agrichemical typically refers to biocides (pesticides including insecticides, herbicides, fungicides and nematicid ...
.

Other reactions
Nitrobenzene
Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced ...
reduction is another reaction catalysed by this compound type:
:
References
{{ChemicalBondsToCarbon