Organomolybdenum Compound on:
[Wikipedia]
[Google]
[Amazon]
 Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements
Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements
 The related complex precursor complex 18 provides even greater opportunities, which is originally designed for the
The related complex precursor complex 18 provides even greater opportunities, which is originally designed for the 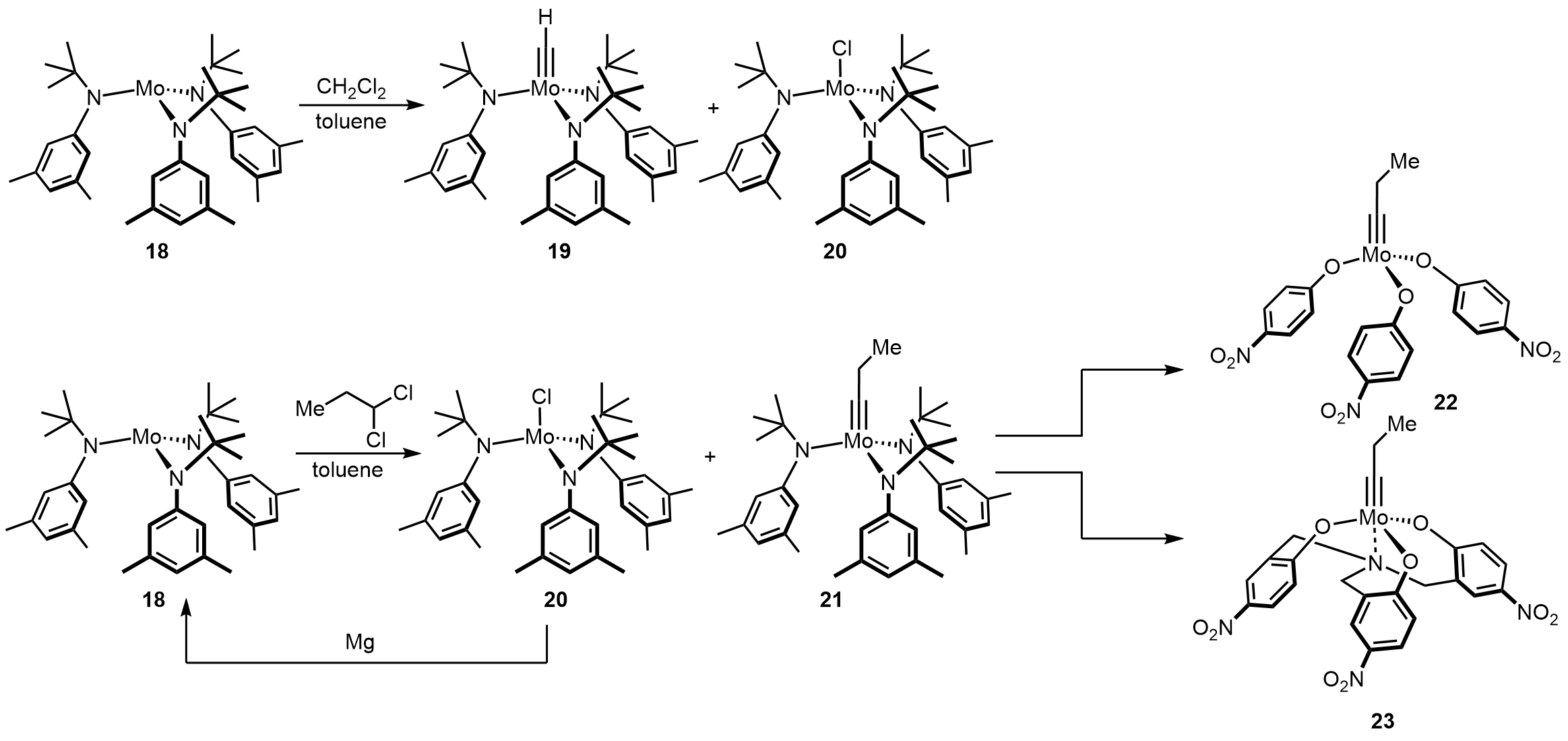 Despite the favorable characteristics of such catalysts, complex 18 must be handled with great care. This compound is not only very sensitive to
Despite the favorable characteristics of such catalysts, complex 18 must be handled with great care. This compound is not only very sensitive to 
 The formal 12-electron count of the W(VI) center in Schrock catalyst represents an appreciable
The formal 12-electron count of the W(VI) center in Schrock catalyst represents an appreciable
 Trisamidomolybdenum(VI) alkylidyne complexes catalyze
Trisamidomolybdenum(VI) alkylidyne complexes catalyze
 Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements
Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with le ...
and tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.
Mo(0) and more reduced states
Molybdenum hexacarbonyl is the precursor to many substituted derivatives. It reacts with organolithium reagents to give anionic acyls which can be O-alkylated to give Fischer carbenes. 144px, Structure of (mesitylene)molybdenum tricarbonyl. Mo(CO)6 reacts with arenes to give piano-stool complexes such as(mesitylene)molybdenum tricarbonyl
(Mesitylene)molybdenum tricarbonyl is an organomolybdenum compound derived from the aromatic compound mesitylene (1,3,5-trimethylbenzene) and molybdenum carbonyl. It exists as pale yellow crystals, which are soluble in organic solvents but decomp ...
. Cycloheptatrienemolybdenum tricarbonyl
Cycloheptatrienemolybdenum tricarbonyl is the organomolybdenum compound with the formula (C7H8)Mo(CO)3. It is a red-orange solid that is soluble in nonpolar organic solvents. The compound has no practical value but is a prototypical complex of c ...
, which is related to (arene)Mo(CO)3, reacts with trityl salts to give the cycloheptatrienyl
In organic chemistry, the tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of 7H7sup>+. Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts ...
complex:
:(C7H8)Mo(CO)3 + (C6H5)3C+ → C7H7)Mo(CO)3sup>+ + (C6H5)3CH
file:CHTMo(CO)3.png, 144px, Structure of Cycloheptatrienemolybdenum tricarbonyl
Cycloheptatrienemolybdenum tricarbonyl is the organomolybdenum compound with the formula (C7H8)Mo(CO)3. It is a red-orange solid that is soluble in nonpolar organic solvents. The compound has no practical value but is a prototypical complex of c ...
.
Reduction of Mo(CO)6 gives [Mo(CO)5]2− which is formally Mo(-II).
CO-free Mo(0) compounds tend to be more reducing and kinetically labile than the carbonyl complexes. Examples include bis(benzene)molybdenum (Mo(C6H6)2) and tris(butadiene)molybdenum. Such compounds can be prepared by metal vapor synthesis and reductive routes from molybdenum(V) chloride.
Mo(II)
Halogenation of Mo(CO)6 gives Mo(II) carbonyl halides, which are also versatile precursors. One large collection of compounds have the formula (C5R5)Mo(CO)3X, derived fromcyclopentadienylmolybdenum tricarbonyl dimer
Cyclopentadienylmolybdenum tricarbonyl dimer is the chemical compound with the formula Cp2Mo2(CO)6, where Cp is C5H5. A dark red solid, it has been the subject of much research although it has no practical uses.
Structure and synthesis
The molec ...
(X = halide, hydride, alkyl).
Treating molybdenum(II) acetate with methyllithium gives Li4 o2(CH3)8
Mo(IV)
With the formula of the type Cp2MoX2molybdocene dichloride
Molybdocene dichloride is the organomolybdenum compound with the formula (hapticity, η5-C5H5)2MoCl2 and IUPAC name dichlorobis(η5-cyclopentadienyl)molybdenum(IV), and is commonly abbreviated as Cp2MoCl2. It is a brownish-green air- and moisture- ...
(X = Cl) and molybdocene dihydride (X = H) are both known as are ansa metallocene analogues.
124px, Molybdocene dihydride.
Mo(V) and Mo(VI)
Mo(CH3)5, Mo(CH3)6, and salts of o(CH3)7sup>− are known. Oxo andimide
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applicati ...
(RN=) ligands are found in several high oxidation state organomolybdenum compounds. The complexes (C5R5)MoO2X are illustrative. Schrock's Mo-based olefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creat ...
catalysts feature molybdenum(VI) centers supported by alkoxide, alkylidene, and imido ligands.
Molybdenum neopentylidyne complexes endowed with sterically demanding phenolates or branched fluorinated alkoxides are catalysts for alkyne metathesis. However, preparation of these catalysts is problematic by the standard Schrock procedure. The trisalkoxide species 17 is active at room temperature.
 The related complex precursor complex 18 provides even greater opportunities, which is originally designed for the
The related complex precursor complex 18 provides even greater opportunities, which is originally designed for the stoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equ ...
cleavage of dinitrogen. In fact, when treating complex 18 with DCM in toluene, the major species formed is a methylidyne complex 19 and a monochloride compound 20. More importantly, the combination of complex 18 and DCM tolerates numerous polar groups. For instance, basic amines and sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds l ...
s, which deactivate the more Lewis acidic complex such as Schrock complex. Following by this original discovery, Moore and co-workers tried higher gem-dichlorides RCHCl2 as activating agents to increase the catalyst lifetime. To reconvert the chloride byproduct, they added magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
in reaction. Moreover, after ligand exchange to an electron deficient ligand such as ''p''-nitrophenol, gave access to a very active catalyst 22, which was effective in many applications, particularly in polymer chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are ...
and material science. On the other hand, alcoholysis of 21 with a tridentate ligand will lead to longer lifetime and better substrate scope.
 Despite the favorable characteristics of such catalysts, complex 18 must be handled with great care. This compound is not only very sensitive to
Despite the favorable characteristics of such catalysts, complex 18 must be handled with great care. This compound is not only very sensitive to oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
and hydrolysis, but even reactive enough to cleave molecular nitrogen.
Molybdenum nitride
In chemistry, a nitride is an inorganic compound of nitrogen. The "nitride" anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occuring. Some nitrides have a find applications, such as wear-resista ...
complexes with Ph3SiO ligands are practical and tolerant precatalyst
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
for alkyne metathesis. This result implied that molybdenum alkylidynes endowed with Ph3SiO ligands must be very active. To further increase the feasibility, stability and activity of these catalysts, they came up with an independent route to directly prepare the alkylidynes instead of their nitrile counterparts. By complexation with 1,10-phenanthroline
1,10-Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. The 1,10 refer to the location of the nitrogen atoms that replace CH's in the hydrocarbon called phenanthrene.
Abbreviated " ...
, an air-stable compound 27 can be formed as precatalyst, which can be activated easily by MnCl2 or ZnCl2 in solvents. As shown below, this route is highly scalable and practical.

Organotungsten compounds
Tungsten analogues of almost all organoMo compounds are known. Some notable examples includehexamethyltungsten
Hexamethyltungsten is the chemical compound W( CH3)6 also written WMe6. Classified as a transition metal alkyl complex, hexamethyltungsten is an air-sensitive, red, crystalline solid at room temperature; however, it is extremely volatile and sub ...
and analogues of Schrock olefin metathesis catalysts.
Many tungsten-based alkyne metathesis
Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. T ...
catalysts are of the general type 3W≡CR Activity is manipulated by the ligands. A typical route to such catalysts entails treatment neopentyl Grignard reagent to tungsten(VI) precursor followed by net alcoholysis of the alkyl ligands. Complex 3 can undergo a ligand exchange with lithium salts to generate Schrock type catalysts (complex 4). Another way to make complex 4 is via cleavage of internal alkyne by W(III) complex, such as 5. Complex 2, as well as 3, is unable to metathesize internal alkynes, the related pathway is shown right. In detail, compound 6 (when X is not OR) will react with two equivalent alkynes to form complex 7. Complex 7 will undergo an "associative path" to generate a metallabenzene The parent metallacyclobenzene has the formula LnM(CH)5. They can be viewed as derivatives of benzene wherein a CH center has been replaced by a transition metal complex. Most metallabenzenes do not feature the M(CH)5 ring itself, but, instead, so ...
complex 8. It will decompose to polymerized compounds or a cyclopentadienyl complex
A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups (, abbreviated as Cp−). Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (''η''5-) bonding mode. The metal–cyclopentadien ...
with a formally reduced tungsten center. Tungstenocenes, or tungsten-containing metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are the metallocene d ...
s, may be formed from these cyclopentadienyl complexes.
 The formal 12-electron count of the W(VI) center in Schrock catalyst represents an appreciable
The formal 12-electron count of the W(VI) center in Schrock catalyst represents an appreciable Lewis acidity
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any spec ...
, which seriously limits the scope of these catalysts. For example, Schrock catalyst is unable to metathesize substrates containing donor or basic sites such as amines, thio ethers or crown ether
In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., . I ...
segments. Acid-sensitive groups such as acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragment ...
s can be destroyed. Replacement of tert-butoxide ligands by fluorinated alkoxides increase the Lewis acidic character. To reach a balance, it is proposed that a heteroleptic
In inorganic chemistry, a homoleptic chemical compound is a metal compound with all ligands identical. The term uses the " homo-" prefix to indicate that something is the same for all. Any metal species which has more than one type of ligand is he ...
push/pull environment around the tungsten center will work.(as shown below) For example, complex 13 is highly active (with loading 1-2 mol% being sufficient) and compatible with many functional groups.
Applications
Mo-based catalysts are useful forolefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creat ...
.
 Trisamidomolybdenum(VI) alkylidyne complexes catalyze
Trisamidomolybdenum(VI) alkylidyne complexes catalyze alkyne metathesis
Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. T ...
.
In the Kauffmann olefination The Kauffmann olefination is a chemical reaction to convert aldehydes and ketones to olefins with a terminal methylene group. This reaction was discovered by the German chemist Thomas Kauffmann and is related to the better known Tebbe olefination or ...
, molybdenum(III) chloride
Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.
Synthesis and structure
Molybdenum(III) chloride is synthesized by the reduction of molybdenum(V) chloride with hydrogen. A higher yield is pro ...
and methyllithium
Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in so ...
form an organometallic complex capable of carbonyl olefination.
References
{{ChemicalBondsToCarbon *