Orbital Motion (quantum) on:
[Wikipedia]
[Google]
[Amazon]
Quantum orbital motion involves the quantum mechanical motion of rigid particles (such as 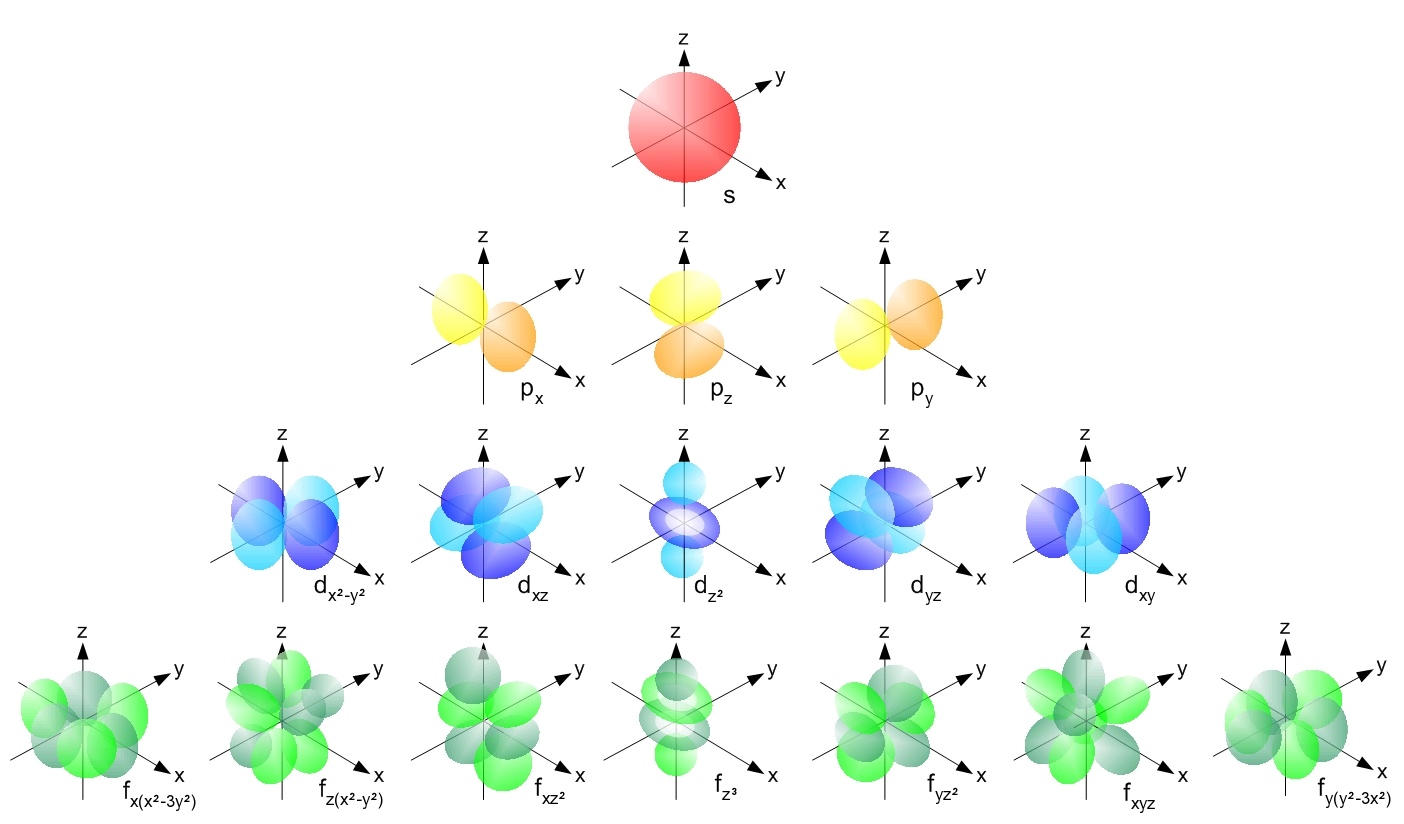
electrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
) about some other mass, or about themselves. In classical mechanics, an object's orbital motion is characterized by its orbital angular momentum
Angular momentum (sometimes called moment of momentum or rotational momentum) is the rotational analog of Momentum, linear momentum. It is an important physical quantity because it is a Conservation law, conserved quantity – the total ang ...
(the angular momentum about the axis of rotation) and spin
Spin or spinning most often refers to:
* Spin (physics) or particle spin, a fundamental property of elementary particles
* Spin quantum number, a number which defines the value of a particle's spin
* Spinning (textiles), the creation of yarn or thr ...
angular momentum, which is the object's angular momentum about its own center of mass. In quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
there are analogous orbital and spin angular momenta which describe the orbital motion of a particle, represented as quantum mechanical operators instead of vectors.
The paradox of Heisenberg's Uncertainty Principle and the wavelike nature of subatomic particles make the exact motion of a particle impossible to represent using classical mechanics. The orbit of an electron about a nucleus is a prime example of quantum orbital motion. While the Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model was a model of the atom that incorporated some early quantum concepts. Developed from 1911 to 1918 by Niels Bohr and building on Ernest Rutherford's nuclear Rutherford model, model, i ...
describes the electron's motion as uniform circular motion, analogous to classical circular motion, in reality its location in space is described by probability functions. Each probability function has a different average energy level, and corresponds to the likelihood of finding the electron in a specific atomic orbital
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
, which are functions representing 3 dimensional regions around the nucleus. The description of orbital motion as probability functions for wavelike particles rather than the specific paths of orbiting bodies is the essential difference between quantum mechanical and classical orbital motion.

Orbital angular momentum
In quantum mechanics, the position of an electron in space is represented by its spatial wave function, and specified by three variables (as with x, y, and z Cartesian coordinates). The square of an electron'swave function
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter) ...
at a given point in space is proportional to the probability of finding it at that point, and each wave function is associated with a particular energy. There are limited allowed wave functions, and thus limited allowed energies of particles in a quantum mechanical system; wave functions are solutions to Schrödinger's equation.
For hydrogen-like atoms, spatial wave function has the following representation:
Electrons do not "orbit" the nucleus in the classical sense of angular momentum, however there is a quantum mechanical analog to the mathematical representation of L = r × p in classical mechanics. In quantum mechanics, these vectors are replaced by operators; the angular momentum operator is defined as the cross product
In mathematics, the cross product or vector product (occasionally directed area product, to emphasize its geometric significance) is a binary operation on two vectors in a three-dimensional oriented Euclidean vector space (named here E), and ...
of the position operator and the momentum operator, which is defined as .
Just as in classical mechanics, the law of conservation of angular momentum
Angular momentum (sometimes called moment of momentum or rotational momentum) is the rotational analog of Momentum, linear momentum. It is an important physical quantity because it is a Conservation law, conserved quantity – the total ang ...
still holds.
Spin
An electron is considered to be a point charge. The motion of this charge about the atomic nucleus produces a magnetic dipole moment that can be oriented in an external magnetic field, as with magnetic resonance. The classical analog to this phenomenon would be a charged particle moving around a circular loop, which constitutes a magnetic dipole. The magnetic moment and angular momentum of this particle would be proportional to each other by the constant , the gyromagnetic ratio. However, unlike bodies in classical mechanics an electron carries an intrinsic property called spin, which creates an additional (spin) magnetic moment. Thetotal angular momentum
In quantum mechanics, the total angular momentum quantum number parametrises the total angular momentum of a given particle, by combining its orbital angular momentum and its intrinsic angular momentum (i.e., its spin).
If s is the particle's ...
of a particle is the sum of both its orbital angular momentum and spin angular momentum.
A particle's spin is generally represented in terms of spin operators. It turns out for particles that make up ordinary matter (protons, neutrons, electrons, quarks, etc.) particles are of spin 1/2.
Only two energy levels (eigenvectors
In linear algebra, an eigenvector ( ) or characteristic vector is a Vector (mathematics and physics), vector that has its direction (geometry), direction unchanged (or reversed) by a given linear map, linear transformation. More precisely, an e ...
of the Hamiltonian) exist for a spin 1/2 state: "up" spin, or +1/2, and "down" spin, or -1/2.
Thus showing that the inherent quantum property of energy quantization is a direct result of electron spin.
Atomic orbitals
Using the formalisms of wave mechanics developed by physicist Erwin Schrödinger in 1926, each electron's distribution is described by a 3-dimensional standing wave. This was motivated by the work of 18th century mathematician Adrien Legendre. The spatial distribution of an electron about a nucleus is represented by three quantum numbers: and . These three numbers describe the electron's atomic orbital, which is the region of space occupied by the electron. Each set of these numbers constitutes a principal shell with a specific number of sub shells, each with a specific number of orbitals. Roughly speaking, the principle quantum number describes the average distance of an electron from the nucleus. The azimuthal quantum number describes the relative shape of the region of space (orbital) occupied by the electron. Finally, The magnetic quantum number describes the relative orientation of the orbital with respect to an applied magnetic field. The allowed values of and depend on the value of .Orbital motion of electrons in hydrogen-like atoms
The simplest physical model of electron behavior in an atom is an electron in hydrogen. For a particle to remain in orbit, it must be bound to its center of rotation by some radial electric potential. In this system, electrons orbiting an atomic nucleus are bound to the nucleus via theCoulomb potential
Electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as electric potential energy per unit of electric charge. More precisely, electric potential is the amount of work (physic ...
, given by . Classically, the energy of the electron orbiting a nucleus would be given as the sum of the kinetic and potential energies. The Bohr model of a hydrogen-like atom
A hydrogen-like atom (or hydrogenic atom) is any atom or ion with a single valence electron. These atoms are isoelectronic with hydrogen. Examples of hydrogen-like atoms include, but are not limited to, hydrogen itself, all alkali metals such as ...
is a classical model of uniform circular motion. Its Hamiltonian is thus written in this way, as . The first term is the kinetic energy of the electron (classically given as , where in quantum mechanics we have replaced classical momentum with the momentum operator . The second term accounts for the Coulomb potential. The Bohr model energies, which are eigenvalues
In linear algebra, an eigenvector ( ) or characteristic vector is a vector that has its direction unchanged (or reversed) by a given linear transformation. More precisely, an eigenvector \mathbf v of a linear transformation T is scaled by a ...
of the Bohr Hamiltonian, are of order , where is the unitless fine-structure constant
In physics, the fine-structure constant, also known as the Sommerfeld constant, commonly denoted by (the Alpha, Greek letter ''alpha''), is a Dimensionless physical constant, fundamental physical constant that quantifies the strength of the el ...
, defined as . (Since is much less than 1, energy corrections with more factors of are significantly smaller order shifts).
However, some revisions must be made to the simplified Bohr model of an electron in the hydrogen atom to account for quantum mechanical effects. These revisions to the electron's motion in a hydrogen atom are some of the most ubiquitous examples of quantum mechanical orbital motion. Ordered by greatest to smallest order of correction to the Bohr energies, the revisions are:
# Motion of the nucleus (of order )
# Fine structure
In atomic physics, the fine structure describes the splitting of the spectral lines of atoms due to electron spin and relativistic corrections to the non-relativistic Schrödinger equation. It was first measured precisely for the hydrogen atom ...
(of order ) or the Zeeman effect
The Zeeman effect () is the splitting of a spectral line into several components in the presence of a static magnetic field. It is caused by the interaction of the magnetic field with the magnetic moment of the atomic electron associated with ...
in the presence of a large magnetic field
## Relativistic correction
## Spin-orbit coupling
# Lamb shift
In physics, the Lamb shift, named after Willis Lamb, is an anomalous difference in energy between two electron orbitals in a hydrogen atom. The difference was not predicted by theory and it cannot be derived from the Dirac equation, which pre ...
(of order ): This is associated with the quantization of the electric field
# Hyperfine splitting
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate electronic energy levels and the resulting splittings in those electronic energy levels of atoms, molecules, and ions, due to electromagnetic multipole int ...
(of order )
For each revision, the Hamiltonian is first rewritten, and then the shifted energy levels are calculated using perturbation theory
In mathematics and applied mathematics, perturbation theory comprises methods for finding an approximate solution to a problem, by starting from the exact solution of a related, simpler problem. A critical feature of the technique is a middle ...
.
Motion of the nucleus
The nucleus is not really perfectly stationary in space; the Coulomb potential attracts it to the electron with equal and opposite force as it exerts on the electron. However, the nucleus is far more massive than the orbiting electron, so its acceleration towards the electron is very small relative to the electron's acceleration towards it, allowing it to be modeled as a also This is accounted for by replacing the mass (m) in the Bohr Hamiltonian with the reduced mass () of the system.Fine structure
# Relativistic correction: The first term in the Hamiltonian represents the kinetic energy of the electron in the atom. However, it comes from the classical expression for kinetic energy . However despite the fact that the electron is moving at relativistic speeds. The relativistic kinetic energy is given as the difference between the electron's total kinetic energy and its rest energy, . Expressing T in terms of the relativistic momentum of the electron and Taylor expanding in powers of the small number , yields a new expression for the kinetic energy which reduces to the classical term to first order: . Giving the lowest-order correction to the Hamiltonian as . In non-denerate perturbation theory, the first order correction to energy levels is given by the expectation value of in the unperturbed state. , and for the unperturbed states the Schrödinger equation reads . Putting these together, the correction to energy is