Nucleoside Phosphoramidite on:
[Wikipedia]
[Google]
[Amazon]
Nucleoside phosphoramidites are derivatives of natural or synthetic
 The common method involves treatment of a protected nucleoside bearing a single free hydroxy group with phosphorodiamidite under the catalytic action of a weak acid. Although some bisamidites were reported as thermally unstable compounds, 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite, the amidite used to prepare commercial nucleoside phosphoramidites is relatively stable. It can be synthesized using a two-step, one-pot procedure and purified by
The common method involves treatment of a protected nucleoside bearing a single free hydroxy group with phosphorodiamidite under the catalytic action of a weak acid. Although some bisamidites were reported as thermally unstable compounds, 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite, the amidite used to prepare commercial nucleoside phosphoramidites is relatively stable. It can be synthesized using a two-step, one-pot procedure and purified by  In the second method, the protected nucleoside is treated with the phosphorochloridite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base).
*
In the second method, the protected nucleoside is treated with the phosphorochloridite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base).
* In the third method, the protected nucleoside is first treated with chloro N,N,N',N'-tetraisopropyl phosphorodiamidite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base) to form a protected nucleoside diamidite. The latter is treated with an alcohol respective to the desired phosphite protecting group, for instance, 2-cyanoethanol, in the presence of a weak acid.
Nucleoside phosphoramidites are purified by
In the third method, the protected nucleoside is first treated with chloro N,N,N',N'-tetraisopropyl phosphorodiamidite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base) to form a protected nucleoside diamidite. The latter is treated with an alcohol respective to the desired phosphite protecting group, for instance, 2-cyanoethanol, in the presence of a weak acid.
Nucleoside phosphoramidites are purified by
 The most important feature of phosphoramidites is their ability to undergo the phosphoramidite coupling reaction that is, to react with nucleophilic groups in the presence of an acidic
The most important feature of phosphoramidites is their ability to undergo the phosphoramidite coupling reaction that is, to react with nucleophilic groups in the presence of an acidic  Phosphoramidites are readily oxidized with weak oxidating reagents, for instance, with aqueous iodine in the presence of weak bases or with
Phosphoramidites are readily oxidized with weak oxidating reagents, for instance, with aqueous iodine in the presence of weak bases or with 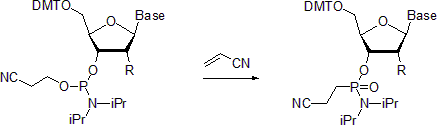 Nucleoside phosphoramidites undergo Michaelis-Arbuzov reaction to form the respective phosphonamidates. One example describes the preparation of phosphonamidates in the presence of
Nucleoside phosphoramidites undergo Michaelis-Arbuzov reaction to form the respective phosphonamidates. One example describes the preparation of phosphonamidates in the presence of
 * In RNA synthesis, the 2'-hydroxy group is protected with TBDMS (''t''-butyldimethylsilyl) group. or with
* In RNA synthesis, the 2'-hydroxy group is protected with TBDMS (''t''-butyldimethylsilyl) group. or with
nucleoside
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotid ...
s. They are used to synthesize oligonucleotide
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, Recombinant DNA, research, and Forensic DNA, forensics. Commonly made in the laboratory by Oligonucleotide synthesis, solid-phase ...
s, relatively short fragments of nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
and their analogs. Nucleoside phosphoramidites were first introduced in 1981 by Beaucage and Caruthers. To avoid undesired side reactions, reactive hydroxy and exocyclic amino groups present in natural or synthetic nucleosides are appropriately protected. As long as a nucleoside analog contains at least one hydroxy group, the use of the appropriate protecting strategy allows one to convert that to the respective phosphoramidite and to incorporate the latter into synthetic nucleic acids. To be incorporated in the middle of an oligonucleotide chain using phosphoramidite strategy, the nucleoside analog must possess two hydroxy groups or, less often, a hydroxy group and another nucleophilic group (amino or mercapto). Examples include, but are not limited to, alternative nucleotides, LNA, morpholino
A Morpholino, also known as a Morpholino oligomer and as a phosphorodiamidate Morpholino oligomer (PMO), is a type of oligomer molecule (colloquially, an oligo) used in molecular biology to modify gene expression. Its molecular structure contains ...
, nucleosides modified at the 2'-position (OMe, protected NH2, F), nucleosides containing non-canonical bases (hypoxanthine
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids, where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-hydroxypurine. Hyp ...
and xanthine
Xanthine ( or , from Ancient Greek for its yellowish-white appearance; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids, as well as in other organisms. Several ...
contained in natural nucleosides inosine
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring (also known as a ribofuranose) via a β-N9-glycosidic bond. It was discovered in 1965 in analysis of RNA transferase.
Inosine is commonly found in tRNAs and is ...
and xanthosine
Xanthosine is a nucleoside derived from xanthine and ribose. It is the biosynthetic precursor to 7-methylxanthosine by the action of 7-methylxanthosine synthase. 7-Methylxanthosine in turn is the precursor to theobromine (active alkaloid in cho ...
, respectively, tricyclic bases such as G-clamp, etc.) or bases derivatized with a fluorescent group or a linker arm.
Preparation
There are three main methods for the preparation of nucleoside phosphoramidites. * The common method involves treatment of a protected nucleoside bearing a single free hydroxy group with phosphorodiamidite under the catalytic action of a weak acid. Although some bisamidites were reported as thermally unstable compounds, 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite, the amidite used to prepare commercial nucleoside phosphoramidites is relatively stable. It can be synthesized using a two-step, one-pot procedure and purified by
The common method involves treatment of a protected nucleoside bearing a single free hydroxy group with phosphorodiamidite under the catalytic action of a weak acid. Although some bisamidites were reported as thermally unstable compounds, 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite, the amidite used to prepare commercial nucleoside phosphoramidites is relatively stable. It can be synthesized using a two-step, one-pot procedure and purified by vacuum
A vacuum (: vacuums or vacua) is space devoid of matter. The word is derived from the Latin adjective (neuter ) meaning "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressur ...
distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixt ...
. An excellent review outlines the use of the latter reagent in preparation of nucleosidic and non-nucleosidic phosphoramidites in great detail.
* In the second method, the protected nucleoside is treated with the phosphorochloridite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base).
*
In the second method, the protected nucleoside is treated with the phosphorochloridite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base).
* In the third method, the protected nucleoside is first treated with chloro N,N,N',N'-tetraisopropyl phosphorodiamidite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base) to form a protected nucleoside diamidite. The latter is treated with an alcohol respective to the desired phosphite protecting group, for instance, 2-cyanoethanol, in the presence of a weak acid.
Nucleoside phosphoramidites are purified by
In the third method, the protected nucleoside is first treated with chloro N,N,N',N'-tetraisopropyl phosphorodiamidite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base) to form a protected nucleoside diamidite. The latter is treated with an alcohol respective to the desired phosphite protecting group, for instance, 2-cyanoethanol, in the presence of a weak acid.
Nucleoside phosphoramidites are purified by column chromatography
Column chromatography in chemistry is a chromatography method used to isolate a single chemical compounds, chemical compound from a mixture. Chromatography is able to separate substances based on differential absorption of compounds to the adsorbe ...
on silica gel
Silica gel is an amorphous and porosity, porous form of silicon dioxide (silica), consisting of an irregular three-dimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain wate ...
. To warrant the stability of the phosphoramidite moiety, it is advisable to equilibrate the column with an eluent containing 3 to 5% of triethylamine and maintain this concentration in the eluent throughout the entire course of the separation. The purity of a phosphoramidite may be assessed by 31P NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic f ...
. As the P(III) atom in a nucleoside phosphoramidite is chiral, it displays two peaks at about 149 ppm corresponding to the two diastereomers of the compound. The potentially present phosphite triester impurity displays peak at 138–140 ppm. H-phosphonate impurities display peaks at 8 and 10 ppm.
Chemical properties of phosphoramidite moiety
Nucleoside phosphoramidites are relatively stable compounds with a prolonged shelf-life when stored as powders under anhydrous conditions in the absence of air at temperatures below 4 °C. The amidites withstand mild basic conditions. In contrast, in the presence of even mild acids, phosphoramidites perish almost instantaneously. The phosphoramidites are relatively stable to hydrolysis under neutral conditions. For instance,half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 2-cyanoethyl 5'-''O''-(4,4'-dimethoxytrityl)thymidine
Thymidine (nucleoside#List of nucleosides and corresponding nucleobases, symbol dT or dThd), also known as deoxythymidine, deoxyribosylthymine, or thymine deoxyriboside, is a pyrimidine nucleoside, deoxynucleoside. Deoxythymidine is the DNA nuc ...
-3'-''O''-(N,N-diisopropylamino)phosphite in 95% aqueous acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
at 25 °C is 200 h.
* The most important feature of phosphoramidites is their ability to undergo the phosphoramidite coupling reaction that is, to react with nucleophilic groups in the presence of an acidic
The most important feature of phosphoramidites is their ability to undergo the phosphoramidite coupling reaction that is, to react with nucleophilic groups in the presence of an acidic azole
Azoles are a class of five-membered heterocyclic compounds containing a nitrogen atom and at least one other non-carbon atom (i.e. nitrogen, sulfur, or oxygen) as part of the ring.
Their names originate from the Hantzsch–Widman nomenclature. Th ...
catalyst, 1''H''-tetrazole, 2-ethylthiotetrazole, 2-benzylthiotetrazole, 4,5-dicyanoimidazole
Imidazole (ImH) is an organic compound with the formula . It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole.
Many natural products, ...
, or a number of similar compounds. The reaction proceeds extremely rapidly. This very feature makes nucleoside phosphoramidites useful intermediates in oligonucleotide synthesis
Oligonucleotide synthesis is the chemical synthesis of relatively short fragments of nucleic acids with defined chemical structure (sequence). The technique is extremely useful in current laboratory practice because it provides a rapid and inexpens ...
. Stereochemically, the phosphoramidite coupling leads to the epimerisation
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization is t ...
(forming of diastereomers
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
) at the P(III) chiral center.
When water is served as a nucleophile, the product is an H-phosphonate diester as shown in Scheme above. Due to the presence of residual water in solvents and reagents, the formation of the latter compound is the most common complication in the preparative use of phosphoramidites, particularly in oligonucleotide synthesis.
* Phosphoramidites are readily oxidized with weak oxidating reagents, for instance, with aqueous iodine in the presence of weak bases or with
Phosphoramidites are readily oxidized with weak oxidating reagents, for instance, with aqueous iodine in the presence of weak bases or with hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
to form the respective phosphoramidates.
Similarly, phosphoramidites react with other chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
s. When brought in contact with a solution of sulfur or a number of compounds collectively referred to as sulfurizing agents, phosphoramidites quantitatively form phosphorothioamidates. The reaction with selenium or selenium derivatives produces phosphoroselenoamidates. In all reactions of this type, the configuration at the phosphorus atom is retained.
* Nucleoside phosphoramidites undergo Michaelis-Arbuzov reaction to form the respective phosphonamidates. One example describes the preparation of phosphonamidates in the presence of
Nucleoside phosphoramidites undergo Michaelis-Arbuzov reaction to form the respective phosphonamidates. One example describes the preparation of phosphonamidates in the presence of acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid. It has a pungent odor of garlic or onions. Its molecular structure consists of a vinyl group () linked to a nitrile (). It is an im ...
. Reportedly, at room temperature the reaction is stereoselective with the retention of configuration at the phosphorus center. In contrast, when carried out at 55 °C, the reaction leads to racemized products.
* Similarly to phosphines and tertiary phosphites, phosphoramidites readily undergo Staudinger reaction
The Staudinger reaction is a chemical reaction of an organic azide with a phosphine or phosphite produces an iminophosphorane. The reaction was discovered by and named after Hermann Staudinger. The reaction follows this stoichiometry:
:R3P + R ...
.
(RO)2P-N(R1)2 + R2-N3 + H2O ---- (RO)2P(=O)-N(R1)2 + R2-NH2 + N2;
Protecting strategy
The naturally occurring nucleotides (nucleoside-3'- or 5'-phosphates) and their phosphodiester analogs are insufficiently reactive to afford an expeditious synthetic preparation of oligonucleotides in high yields. The selectivity and the rate of the formation of internucleosidic linkages are dramatically improved by using 3'-''O''-(''N'',''N''-diisopropyl phosphoramidite) derivatives of nucleosides (nucleoside phosphoramidites) that serve as building blocks in phosphite triester methodology. To prevent undesired side reactions, all other functional groups present in nucleosides must be rendered unreactive (protected) by attachingprotecting groups
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep synthesis, multistep organic ...
. Upon the completion of the oligonucleotide chain assembly, all the protecting groups are removed to yield the desired oligonucleotides. Below, the protecting groups currently used in commercially available and most common nucleoside phosphoramidite building blocks are briefly reviewed:
* The 5'-hydroxyl group is protected by an acid-labile DMT
Dimethyltryptamine (DMT), also known as ''N'',''N''-dimethyltryptamine (''N'',''N''-DMT), is a serotonergic hallucinogen and investigational drug of the tryptamine family that occurs naturally in many plants and animals, including humans. D ...
(4,4'-dimethoxytrityl) group.
* Thymine
Thymine () (symbol T or Thy) is one of the four nucleotide bases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine ...
and uracil
Uracil () (nucleoside#List of nucleosides and corresponding nucleobases, symbol U or Ura) is one of the four nucleotide bases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via ...
, nucleic bases of thymidine
Thymidine (nucleoside#List of nucleosides and corresponding nucleobases, symbol dT or dThd), also known as deoxythymidine, deoxyribosylthymine, or thymine deoxyriboside, is a pyrimidine nucleoside, deoxynucleoside. Deoxythymidine is the DNA nuc ...
and uridine
Uridine (symbol U or Urd) is a glycosylated pyrimidine analog containing uracil attached to a ribose ring (or more specifically, a ribofuranose) via a β-N1- glycosidic bond. The analog is one of the five standard nucleosides which make up nuc ...
, respectively, do not have exocyclic amino groups and hence do not require any protection. In contrast, nucleic bases adenine
Adenine (, ) (nucleoside#List of nucleosides and corresponding nucleobases, symbol A or Ade) is a purine nucleotide base that is found in DNA, RNA, and Adenosine triphosphate, ATP. Usually a white crystalline subtance. The shape of adenine is ...
, cytosine
Cytosine () (symbol C or Cyt) is one of the four nucleotide bases found in DNA and RNA, along with adenine, guanine, and thymine ( uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attac ...
, and guanine
Guanine () (symbol G or Gua) is one of the four main nucleotide bases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine ( uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside ...
bear the exocyclic amino groups, which are reactive with the activated phosphoramidites under the conditions of the coupling reaction. Although, at the expense of additional steps in the synthetic cycle, the oligonucleotide chain assembly may be carried out using phosphoramidites with unprotected amino groups, most often these are kept permanently protected over the entire length of the oligonucleotide chain assembly. The protection of the exocyclic amino groups must be orthogonal to that of the 5'-hydroxy group because the latter is removed at the end of each synthetic cycle. The simplest to implement and hence the most widely accepted is the strategy where the exocyclic amino groups bear a base-labile protection. Most often, two protection schemes are used.
* In the first, the standard and more robust scheme (Figure), Bz (benzoyl) protection is used for A, dA, C, dC, G, and dG are protected with isobutyryl group. More recently, Ac (acetyl) group is often used to protect C and dC as shown in Figure.
* In the second, mild protection scheme, A and dA are protected with isobutyryl or phenoxyacetyl groups (PAC). C and dC bear acetyl protection, and G and dG are protected with 4-isopropylphenoxyacetyl (i-Pr-PAC) or dimethylformamidino (dmf) groups. Mild protecting groups are removed more readily than the standard protecting groups. However, the phosphoramidites bearing these groups are less stable when stored in solution.
* The phosphite group is protected by a base-labile 2-cyanoethyl group. Once a phosphoramidite has been coupled to the solid support-bound oligonucleotide and the phosphite moieties have been converted to the P(V) species, the presence of the phosphate protection is not mandatory for the successful conducting of further coupling reactions.
 * In RNA synthesis, the 2'-hydroxy group is protected with TBDMS (''t''-butyldimethylsilyl) group. or with
* In RNA synthesis, the 2'-hydroxy group is protected with TBDMS (''t''-butyldimethylsilyl) group. or with TOM
Tom or TOM may refer to:
* Tom (given name), including a list of people and fictional characters with the name.
Arts and entertainment Film and television
* ''Tom'' (1973 film), or ''The Bad Bunch'', a blaxploitation film
* ''Tom'' (2002 film) ...
(tri-''iso''-propylsilyloxymethyl) group, both being removable by treatment with fluoride ion.
* The phosphite moiety also bears a diisopropylamino (''i''Pr2N) group reactive under acidic conditions. On activation, the diisopropylamino group leaves, to be substituted by the 5'-hydroxy group of the support-bound oligonucleotide.
See also
*DNA synthesis
DNA synthesis is the natural or artificial creation of deoxyribonucleic acid (DNA) molecules. DNA is a macromolecule made up of nucleotide units, which are linked by covalent bonds and hydrogen bonds, in a repeating structure. DNA synthesis occu ...
*Nucleic acid analogues
Nucleic acid analogues are compounds which are analogous (structurally similar) to naturally occurring RNA and DNA, used in medicine and in molecular biology research. Nucleic acids are chains of nucleotides, which are composed of three parts: ...
*Oligonucleotide synthesis
Oligonucleotide synthesis is the chemical synthesis of relatively short fragments of nucleic acids with defined chemical structure (sequence). The technique is extremely useful in current laboratory practice because it provides a rapid and inexpens ...
References
Further reading
*Comprehensive Natural Products Chemistry, Volume 7: DNA and Aspects of Molecular Biology. Kool, Eric T.; Editor. Neth. (1999), 733 pp. Publisher: (Elsevier, Amsterdam, Neth.) * * * *Beaucage, S L. "Oligodeoxyribonucleotides synthesis. Phosphoramidite approach. Methods in Molecular Biology (Totowa, NJ, United States) (1993), 20 (Protocols for Oligonucleotides and Analogs), 33–61. * *Brown T., Brown D. J. S. 1991. In Oligonucleotides and Analogues. A Practical Approach, ed. F Eckstein, pp. 1 – 24. Oxford: IRL {{organophosphorus Phosphorus-nitrogen compounds pl:Amidofosforyny