Nobelium on:
[Wikipedia]
[Google]
[Amazon]
Nobelium is a synthetic chemical element; it has
 The discovery of element 102 was a complicated process and was claimed by groups from
The discovery of element 102 was a complicated process and was claimed by groups from  In 1969, the Dubna team carried out chemical experiments on element 102 and concluded that it behaved as the heavier homologue of ytterbium. The Russian scientists proposed the name ''joliotium'' (Jo) for the new element after Irène Joliot-Curie, who had recently died, creating an element naming controversy that would not be resolved for several decades, with each group using its own proposed names.
In 1992, the
In 1969, the Dubna team carried out chemical experiments on element 102 and concluded that it behaved as the heavier homologue of ytterbium. The Russian scientists proposed the name ''joliotium'' (Jo) for the new element after Irène Joliot-Curie, who had recently died, creating an element naming controversy that would not be resolved for several decades, with each group using its own proposed names.
In 1992, the
 In the
In the
Chart of Nuclides
. nndc.bnl.gov
Los Alamos National Laboratory – Nobelium
at ''
symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
No and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
102. It is named after Alfred Nobel
Alfred Bernhard Nobel ( ; ; 21 October 1833 – 10 December 1896) was a Swedish chemist, inventor, engineer, and businessman. He is known for inventing dynamite, as well as having bequeathed his fortune to establish the Nobel Prizes. He also m ...
, the inventor of dynamite
Dynamite is an explosive made of nitroglycerin, sorbents (such as powdered shells or clay), and Stabilizer (chemistry), stabilizers. It was invented by the Swedish people, Swedish chemist and engineer Alfred Nobel in Geesthacht, Northern German ...
and benefactor of science. A radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
, it is the tenth transuranium element, the second transfermium, and is the penultimate member of the actinide series. Like all elements with atomic number over 100, nobelium can only be produced in particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel electric charge, charged particles to very high speeds and energies to contain them in well-defined particle beam, beams. Small accelerators are used for fundamental ...
s by bombarding lighter elements with charged particles. A total of twelve nobelium isotopes are known to exist; the most stable is 259No with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 58 minutes, but the shorter-lived 255No (half-life 3.1 minutes) is most commonly used in chemistry because it can be produced on a larger scale.
Chemistry experiments have confirmed that nobelium behaves as a heavier homolog to ytterbium in the periodic table. The chemical properties of nobelium are not completely known: they are mostly only known in aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
. Before nobelium's discovery, it was predicted that it would show a stable +2 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
as well as the +3 state characteristic of the other actinides; these predictions were later confirmed, as the +2 state is much more stable than the +3 state in aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water ...
and it is difficult to keep nobelium in the +3 state.
In the 1950s and 1960s, many claims of the discovery of nobelium were made from laboratories in Sweden
Sweden, formally the Kingdom of Sweden, is a Nordic countries, Nordic country located on the Scandinavian Peninsula in Northern Europe. It borders Norway to the west and north, and Finland to the east. At , Sweden is the largest Nordic count ...
, the Soviet Union
The Union of Soviet Socialist Republics. (USSR), commonly known as the Soviet Union, was a List of former transcontinental countries#Since 1700, transcontinental country that spanned much of Eurasia from 1922 until Dissolution of the Soviet ...
, and the United States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
. Although the Swedish scientists soon retracted their claims, the priority of the discovery and therefore the naming of the element was disputed between Soviet and American scientists. It was not until 1992 that the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC) credited the Soviet team with the discovery. Even so, nobelium, the Swedish proposal, was retained as the name of the element due to its long-standing use in the literature.
Introduction
Discovery
 The discovery of element 102 was a complicated process and was claimed by groups from
The discovery of element 102 was a complicated process and was claimed by groups from Sweden
Sweden, formally the Kingdom of Sweden, is a Nordic countries, Nordic country located on the Scandinavian Peninsula in Northern Europe. It borders Norway to the west and north, and Finland to the east. At , Sweden is the largest Nordic count ...
, the United States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
, and the Soviet Union
The Union of Soviet Socialist Republics. (USSR), commonly known as the Soviet Union, was a List of former transcontinental countries#Since 1700, transcontinental country that spanned much of Eurasia from 1922 until Dissolution of the Soviet ...
. The first complete and incontrovertible report of its detection only came in 1966 from the Joint Institute of Nuclear Research at Dubna
Dubna ( rus, Дубна́, p=dʊbˈna) is a town in Moscow Oblast, Russia. It has a status of '' naukograd'' (i.e. town of science), being home to the Joint Institute for Nuclear Research, an international nuclear physics research center and o ...
(then in the Soviet Union). (Note: for Part I see Pure and Applied Chemistry, vol. 63, no. 6, pp. 879–886, 1991)
The first announcement of the discovery of element 102 was announced by physicists at the Nobel Institute for Physics in Sweden in 1957. The team reported that they had bombarded a curium target with carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth.
Detection by mass spectrometry
A m ...
ions for twenty-five hours in half-hour intervals. Between bombardments, ion-exchange chemistry was performed on the target. Twelve out of the fifty bombardments contained samples emitting (8.5 ± 0.1) MeV alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s, which were in drops which eluted earlier than fermium (atomic number ''Z'' = 100) and californium
Californium is a synthetic chemical element; it has symbol Cf and atomic number 98. It was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) by bombarding curium with al ...
(''Z'' = 98). The half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
reported was 10 minutes and was assigned to either 251102 or 253102, although the possibility that the alpha particles observed were from a presumably short-lived mendelevium
Mendelevium is a synthetic chemical element; it has symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced ...
(''Z'' = 101) isotope created from the electron capture of element 102 was not excluded. The team proposed the name ''nobelium'' (No) for the new element, which was immediately approved by IUPAC, a decision which the Dubna group characterized in 1968 as being hasty.
In 1958, scientists at the Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory (LBNL, Berkeley Lab) is a Federally funded research and development centers, federally funded research and development center in the Berkeley Hills, hills of Berkeley, California, United States. Established i ...
repeated the experiment. The Berkeley team, consisting of Albert Ghiorso
Albert Ghiorso (July 15, 1915 – December 26, 2010) was an American nuclear scientist and co-discoverer of a record 12 chemical elements on the periodic table. His research career spanned six decades, from the early 1940s to the late 1990s.
Biog ...
, Glenn T. Seaborg, John R. Walton and Torbjørn Sikkeland, used the new heavy- ion linear accelerator
A linear particle accelerator (often shortened to linac) is a type of particle accelerator that accelerates charged subatomic particles or ions to a high speed by subjecting them to a series of oscillating electric potentials along a linear ...
(HILAC) to bombard a curium target (95% 244Cm and 5% 246Cm) with 13C and 12C ions. They were unable to confirm the 8.5 MeV activity claimed by the Swedes but were instead able to detect decays from fermium-250, supposedly the daughter of 254102 (produced from the curium-246), which had an apparent half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of ~3 s. Probably this assignment was also wrong, as later 1963 Dubna work showed that the half-life of 254No is significantly longer (about 50 s). It is more likely that the observed alpha decays did not come from element 102, but rather from 250mFm.
In 1959, the Swedish team attempted to explain the Berkeley team's inability to detect element 102 in 1958, maintaining that they did discover it. However, later work has shown that no nobelium isotopes lighter than 259No (no heavier isotopes could have been produced in the Swedish experiments) with a half-life over 3 minutes exist, and that the Swedish team's results are most likely from thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
-225, which has a half-life of 8 minutes and quickly undergoes triple alpha decay to polonium-213, which has a decay energy of 8.53612 MeV. This hypothesis is lent weight by the fact that thorium-225 can easily be produced in the reaction used and would not be separated out by the chemical methods used. Later work on nobelium also showed that the divalent state is more stable than the trivalent one and hence that the samples emitting the alpha particles could not have contained nobelium, as the divalent nobelium would not have eluted with the other trivalent actinides. Thus, the Swedish team later retracted their claim and associated the activity to background effects.
In 1959, the team continued their studies and claimed that they were able to produce an isotope that decayed predominantly by emission of an 8.3 MeV alpha particle, with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 3 s with an associated 30% spontaneous fission branch. The activity was initially assigned to 254102 but later changed to 252102. However, they also noted that it was not certain that element 102 had been produced due to difficult conditions. The Berkeley team decided to adopt the proposed name of the Swedish team, "nobelium", for the element.
: + → → + 4
Meanwhile, in Dubna, experiments were carried out in 1958 and 1960 aiming to synthesize element 102 as well. The first 1958 experiment bombarded plutonium-239 and -241 with oxygen-16 ions. Some alpha decays with energies just over 8.5 MeV were observed, and they were assigned to 251,252,253102, although the team wrote that formation of isotopes from lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
or bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
impurities (which would not produce nobelium) could not be ruled out. While later 1958 experiments noted that new isotopes could be produced from mercury, thallium
Thallium is a chemical element; it has Symbol (chemistry), symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Che ...
, lead, or bismuth impurities, the scientists still stood by their conclusion that element 102 could be produced from this reaction, mentioning a half-life of under 30 seconds and a decay energy of (8.8 ± 0.5) MeV. Later 1960 experiments proved that these were background effects. 1967 experiments also lowered the decay energy to (8.6 ± 0.4) MeV, but both values are too high to possibly match those of 253No or 254No. The Dubna team later stated in 1970 and again in 1987 that these results were not conclusive.
In 1961, Berkeley scientists claimed the discovery of element 103 in the reaction of californium with boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
and carbon ions. They claimed the production of the isotope 257103, and also claimed to have synthesized an alpha decaying isotope of element 102 that had a half-life of 15 s and alpha decay energy 8.2 MeV. They assigned this to 255102 without giving a reason for the assignment. The values do not agree with those now known for 255No, although they do agree with those now known for 257No, and while this isotope probably played a part in this experiment, its discovery was inconclusive.
Work on element 102 also continued in Dubna, and in 1964, experiments were carried out there to detect alpha-decay daughters of element 102 isotopes by synthesizing element 102 from the reaction of a uranium-238 target with neon
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of ...
ions. The products were carried along a silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
catcher foil and purified chemically, and the isotopes 250Fm and 252Fm were detected. The yield of 252Fm was interpreted as evidence that its parent 256102 was also synthesized: as it was noted that 252Fm could also be produced directly in this reaction by the simultaneous emission of an alpha particle with the excess neutrons, steps were taken to ensure that 252Fm could not go directly to the catcher foil. The half-life detected for 256102 was 8 s, which is much higher than the more modern 1967 value of (3.2 ± 0.2) s. Further experiments were conducted in 1966 for 254102, using the reactions 243 Am(15 N,4n)254102 and 238U(22Ne,6n)254102, finding a half-life of (50 ± 10) s: at that time the discrepancy between this value and the earlier Berkeley value was not understood, although later work proved that the formation of the isomer 250mFm was less likely in the Dubna experiments than at the Berkeley ones. In hindsight, the Dubna results on 254102 were probably correct and can be now considered a conclusive detection of element 102.
One more very convincing experiment from Dubna was published in 1966 (though it was submitted in 1965), again using the same two reactions, which concluded that 254102 indeed had a half-life much longer than the 3 seconds claimed by Berkeley. Later work in 1967 at Berkeley and 1971 at the Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a federally funded research and development centers, federally funded research and development center in Oak Ridge, Tennessee, United States. Founded in 1943, the laboratory is sponsored by the United Sta ...
fully confirmed the discovery of element 102 and clarified earlier observations. In December 1966, the Berkeley group repeated the Dubna experiments and fully confirmed them, and used this data to finally assign correctly the isotopes they had previously synthesized but could not yet identify at the time. Thus they claimed to have discovered nobelium in 1958 to 1961.
: + → → + 6
 In 1969, the Dubna team carried out chemical experiments on element 102 and concluded that it behaved as the heavier homologue of ytterbium. The Russian scientists proposed the name ''joliotium'' (Jo) for the new element after Irène Joliot-Curie, who had recently died, creating an element naming controversy that would not be resolved for several decades, with each group using its own proposed names.
In 1992, the
In 1969, the Dubna team carried out chemical experiments on element 102 and concluded that it behaved as the heavier homologue of ytterbium. The Russian scientists proposed the name ''joliotium'' (Jo) for the new element after Irène Joliot-Curie, who had recently died, creating an element naming controversy that would not be resolved for several decades, with each group using its own proposed names.
In 1992, the IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
- IUPAP Transfermium Working Group (TWG) reassessed the claims of discovery and concluded that only the Dubna work from 1966 correctly detected and assigned decays to nuclei with atomic number 102 at the time. The Dubna team are therefore officially recognised as the discoverers of nobelium, although it is possible that it was detected at Berkeley in 1959. This decision was criticized by Berkeley the following year, calling the reopening of the cases of elements 101 to 103 a "futile waste of time", while Dubna agreed with IUPAC's decision.
In 1994, as part of an attempted resolution to the element naming controversy, IUPAC ratified names for elements 101–109. For element 102, it ratified the name ''nobelium'' (No) on the basis that it had become entrenched in the literature over the course of 30 years and that Alfred Nobel
Alfred Bernhard Nobel ( ; ; 21 October 1833 – 10 December 1896) was a Swedish chemist, inventor, engineer, and businessman. He is known for inventing dynamite, as well as having bequeathed his fortune to establish the Nobel Prizes. He also m ...
should be commemorated in this fashion. Because of outcry over the 1994 names, which mostly did not respect the choices of the discoverers, a comment period ensued, and in 1995 IUPAC named element 102 ''flerovium'' (Fl) as part of a new proposal, after either Georgy Flyorov or his eponymous Flerov Laboratory of Nuclear Reactions. This proposal was also not accepted, and in 1997 the name ''nobelium'' was restored. Today the name ''flerovium'', with the same symbol, refers to element 114.
Characteristics
Physical
 In the
In the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
, nobelium is located to the right of the actinide mendelevium
Mendelevium is a synthetic chemical element; it has symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced ...
, to the left of the actinide lawrencium, and below the lanthanide ytterbium. Nobelium metal has not yet been prepared in bulk quantities, and bulk preparation is currently impossible. Nevertheless, a number of predictions and some preliminary experimental results have been done regarding its properties.
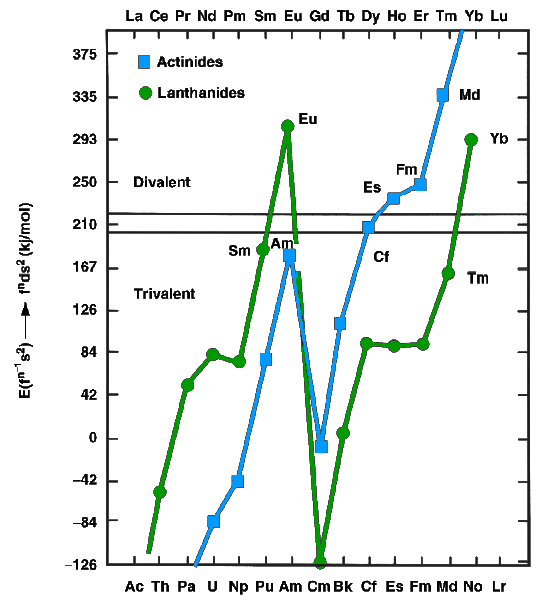
The lanthanides and actinides, in the metallic state, can exist as either divalent (such as europium
Europium is a chemical element; it has symbol Eu and atomic number 63. It is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. Europium is the most chemically reactive, least dense, and soft ...
and ytterbium) or trivalent (most other lanthanides) metals. The former have f''n''s2 configurations, whereas the latter have f''n''−1d1s2 configurations. In 1975, Johansson and Rosengren examined the measured and predicted values for the cohesive energies ( enthalpies of crystallization) of the metallic lanthanides and actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
s, both as divalent and trivalent metals. The conclusion was that the increased binding energy of the nf136d17s2 configuration over the nf147s2 configuration for nobelium was not enough to compensate for the energy needed to promote one 5f electron to 6d, as is true also for the very late actinides: thus einsteinium, fermium, mendelevium
Mendelevium is a synthetic chemical element; it has symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced ...
, and nobelium were expected to be divalent metals, although for nobelium this prediction has not yet been confirmed. The increasing predominance of the divalent state well before the actinide series concludes is attributed to the relativistic stabilization of the 5f electrons, which increases with increasing atomic number: an effect of this is that nobelium is predominantly divalent instead of trivalent, unlike all the other lanthanides and actinides. In 1986, nobelium metal was estimated to have an enthalpy of sublimation between 126 kJ/mol, a value close to the values for einsteinium, fermium, and mendelevium and supporting the theory that nobelium would form a divalent metal. Like the other divalent late actinides (except the once again trivalent lawrencium), metallic nobelium should assume a face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
crystal structure. Divalent nobelium metal should have a metallic radius of around 197 pm. Nobelium's melting point has been predicted to be 800 °C, the same value as that estimated for the neighboring element mendelevium. Its density is predicted to be around 9.9 ± 0.4 g/cm3.
Chemical
The chemistry of nobelium is incompletely characterized and is known only in aqueous solution, in which it can take on the +3 or +2oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
s, the latter being more stable. It was largely expected before the discovery of nobelium that in solution, it would behave like the other actinides, with the trivalent state being predominant; however, Seaborg predicted in 1949 that the +2 state would also be relatively stable for nobelium, as the No2+ ion would have the ground-state electron configuration nf14, including the stable filled 5f14 shell. It took nineteen years before this prediction was confirmed.
In 1967, experiments were conducted to compare nobelium's chemical behavior to that of terbium
Terbium is a chemical element; it has Symbol (chemistry), symbol Tb and atomic number 65. It is a silvery-white, rare earth element, rare earth metal that is malleable and ductile. The ninth member of the lanthanide series, terbium is a fairly ele ...
, californium
Californium is a synthetic chemical element; it has symbol Cf and atomic number 98. It was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) by bombarding curium with al ...
, and fermium. All four elements were reacted with chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
and the resulting chlorides were deposited along a tube, along which they were carried by a gas. It was found that the nobelium chloride produced was strongly adsorbed on solid surfaces, proving that it was not very volatile, like the chlorides of the other three investigated elements. However, both NoCl2 and NoCl3 were expected to exhibit nonvolatile behavior and hence this experiment was inconclusive as to what the preferred oxidation state of nobelium was. Determination of nobelium's favoring of the +2 state had to wait until the next year, when cation-exchange chromatography and coprecipitation experiments were carried out on around fifty thousand 255No atoms, finding that it behaved differently from the other actinides and more like the divalent alkaline earth metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar p ...
s. This proved that in aqueous solution, nobelium is most stable in the divalent state when strong oxidizers are absent. Later experimentation in 1974 showed that nobelium eluted with the alkaline earth metals, between Ca2+ and Sr2+. Nobelium is the only known f-block element for which the +2 state is the most common and stable one in aqueous solution. This occurs because of the large energy gap between the 5f and 6d orbitals at the end of the actinide series.
It is expected that the relativistic stabilization of the 7s subshell greatly destabilizes nobelium dihydride, NoH2, and relativistic stabilisation of the 7p1/2 spinor over the 6d3/2 spinor mean that excited states in nobelium atoms have 7s and 7p contribution instead of the expected 6d contribution. The long No–H distances in the NoH2 molecule and the significant charge transfer lead to extreme ionicity with a dipole moment of 5.94 D for this molecule. In this molecule, nobelium is expected to exhibit main-group-like behavior, specifically acting like an alkaline earth metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar p ...
with its ''n''s2 valence shell configuration and core-like 5f orbitals.
Nobelium's complexing ability with chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
ions is most similar to that of barium
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
, which complexes rather weakly. Its complexing ability with citrate, oxalate, and acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
in an aqueous solution of 0.5 M ammonium nitrate
Ammonium nitrate is a chemical compound with the formula . It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, but does not form hydrates. It is predominantly us ...
is between that of calcium and strontium, although it is somewhat closer to that of strontium.
The standard reduction potential of the ''E''°(No3+→No2+) couple was estimated in 1967 to be between +1.4 and +1.5 V; it was later found in 2009 to be only about +0.75 V. The positive value shows that No2+ is more stable than No3+ and that No3+ is a good oxidizing agent. While the quoted values for the ''E''°(No2+→No0) and ''E''°(No3+→No0) vary among sources, the accepted standard estimates are −2.61 and −1.26 V. It has been predicted that the value for the ''E''°(No4+→No3+) couple would be +6.5 V. The Gibbs energies of formation for No3+ and No2+ are estimated to be −342 and −480 kJ/mol, respectively.
Atomic
A nobelium atom has 102 electrons. They are expected to be arranged in the configuration nf147s2 (ground stateterm symbol
In atomic physics, a term symbol is an abbreviated description of the total spin and orbital angular momentum quantum numbers of the electrons in a multi-electron atom. So while the word ''symbol'' suggests otherwise, it represents an actual ''valu ...
1S0), although experimental verification of this electron configuration had not yet been made as of 2006. The sixteen electrons in the 5f and 7s subshells are valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s. In forming compounds, three valence electrons may be lost, leaving behind a nf13 core: this conforms to the trend set by the other actinides with their nf''n'' electron configurations in the tripositive state. Nevertheless, it is more likely that only two valence electrons are lost, leaving behind a stable nf14 core with a filled 5f14 shell. The first ionization potential
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. The first ionization energy is quantitatively expressed as
:X(g) ...
of nobelium was measured to be at most (6.65 ± 0.07) eV in 1974, based on the assumption that the 7s electrons would ionize before the 5f ones; this value has not yet been refined further due to nobelium's scarcity and high radioactivity.Lide, David R. (editor), ''CRC Handbook of Chemistry and Physics, 84th Edition'', CRC Press, Boca Raton (FL), 2003, section 10, ''Atomic, Molecular, and Optical Physics; Ionization Potentials of Atoms and Atomic Ions'' The ionic radius of hexacoordinate and octacoordinate No3+ had been preliminarily estimated in 1978 to be around 90 and 102 pm respectively; the ionic radius of No2+ has been experimentally found to be 100 pm to two significant figures. The enthalpy of hydration of No2+ has been calculated as 1486 kJ/mol.
Isotopes
Fourteen isotopes of nobelium are known, withmass number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is appro ...
s 248–260 and 262; all are radioactive. Additionally, nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have Half-life, half-lives of ...
s are known for mass numbers 250, 251, 253, and 254. Of these, the longest-lived isotope is 259No with a half-life of 58 minutes, and the longest-lived isomer is 251mNo with a half-life of 1.7 seconds. However, the still undiscovered isotope 261No is predicted to have a still longer half-life of 3 hours. Additionally, the shorter-lived 255No (half-life 3.1 minutes) is more often used in chemical experimentation because it can be produced in larger quantities from irradiation of californium-249 with carbon-12
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon ( carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-1 ...
ions. After 259No and 255No, the next most stable nobelium isotopes are 253No (half-life 1.62 minutes), 254No (51 second
The second (symbol: s) is a unit of time derived from the division of the day first into 24 hours, then to 60 minutes, and finally to 60 seconds each (24 × 60 × 60 = 86400). The current and formal definition in the International System of U ...
s), 257No (25 seconds), 256No (2.91 seconds), and 252No (2.57 seconds). All of the remaining nobelium isotopes have half-lives that are less than a second, and the shortest-lived known nobelium isotope (248No) has a half-life of less than 2 microsecond
A microsecond is a unit of time in the International System of Units (SI) equal to one millionth (0.000001 or 10−6 or ) of a second. Its symbol is μs, sometimes simplified to us when Unicode is not available.
A microsecond is to one second, ...
s. The isotope 254No is especially interesting theoretically as it is in the middle of a series of prolate nuclei from 231 Pa to 279 Rg, and the formation of its nuclear isomers (of which two are known) is controlled by proton orbitals such as 2f5/2 which come just above the spherical proton shell; it can be synthesized in the reaction of 208Pb with 48Ca.
The half-lives of nobelium isotopes increase smoothly from 250No to 253No. However, a dip appears at 254No, and beyond this the half-lives of even-even nobelium isotopes drop sharply as spontaneous fission becomes the dominant decay mode. For example, the half-life of 256No is almost three seconds, but that of 258No is only 1.2 milliseconds. This shows that at nobelium, the mutual repulsion of protons poses a limit to the region of long-lived nuclei in the actinide
The actinide () or actinoid () series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. Number 103, lawrencium, is also generally included despite being part ...
series. The even-odd nobelium isotopes mostly continue to have longer half-lives as their mass numbers increase, with a dip in the trend at 257No.
Preparation and purification
The isotopes of nobelium are mostly produced by bombarding actinide targets (uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
, plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
, curium, californium
Californium is a synthetic chemical element; it has symbol Cf and atomic number 98. It was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) by bombarding curium with al ...
, or einsteinium), with the exception of nobelium-262, which is produced as the daughter of lawrencium-262. The most commonly used isotope, 255No, can be produced from bombarding curium-248 or californium-249 with carbon-12: the latter method is more common. Irradiating a 350 μg
In the metric system, a microgram or microgramme is a Physical unit, unit of mass equal to one millionth () of a gram. The unit symbol is μg according to the International System of Units (SI); the recommended symbol in the United States and Uni ...
cm−2 target of californium-249 with three trillion 73 MeV carbon-12 ions per second for ten minutes can produce around 1200 nobelium-255 atoms.
Once the nobelium-255 is produced, it can be separated out similarly as used to purify the neighboring actinide mendelevium. The recoil momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
of the produced nobelium-255 atoms is used to bring them physically far away from the target from which they are produced, bringing them onto a thin foil of metal (usually beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with ...
, aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
, platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
, or gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
) just behind the target in a vacuum: this is usually combined by trapping the nobelium atoms in a gas atmosphere (frequently helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
), and carrying them along with a gas jet from a small opening in the reaction chamber. Using a long capillary tube, and including potassium chloride aerosols in the helium gas, the nobelium atoms can be transported over tens of meter
The metre (or meter in US spelling; symbol: m) is the base unit of length in the International System of Units (SI). Since 2019, the metre has been defined as the length of the path travelled by light in vacuum during a time interval of of ...
s. The thin layer of nobelium collected on the foil can then be removed with dilute acid without completely dissolving the foil. The nobelium can then be isolated by exploiting its tendency to form the divalent state, unlike the other trivalent actinides: under typically used elution conditions ( bis-(2-ethylhexyl) phosphoric acid (HDEHP) as stationary organic phase and 0.05 M hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
as mobile aqueous phase, or using 3 M hydrochloric acid as an eluant from cation-exchange resin columns), nobelium will pass through the column and elute while the other trivalent actinides remain on the column. However, if a direct "catcher" gold foil is used, the process is complicated by the need to separate out the gold using anion-exchange chromatography
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it ...
before isolating the nobelium by elution from chromatographic extraction columns using HDEHP.
Notes
References
Bibliography
* * * * * *External links
Chart of Nuclides
. nndc.bnl.gov
Los Alamos National Laboratory – Nobelium
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
{{Good article
Chemical elements
Chemical elements with face-centered cubic structure
Actinides
Synthetic elements
Alfred Nobel