nitrene on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 :A nitrene intermediate is suspected in this C–H insertion involving an
:A nitrene intermediate is suspected in this C–H insertion involving an  * Nitrene cycloaddition. With
* Nitrene cycloaddition. With  :In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
::
:In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
::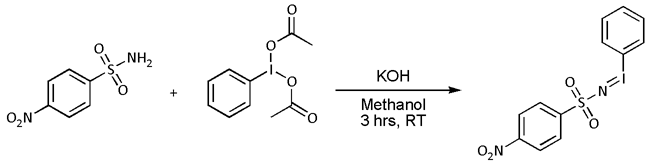 :Nitrene transfer takes place next:
::
:Nitrene transfer takes place next:
:: :In this particular reaction both the '' cis''-
:In this particular reaction both the '' cis''-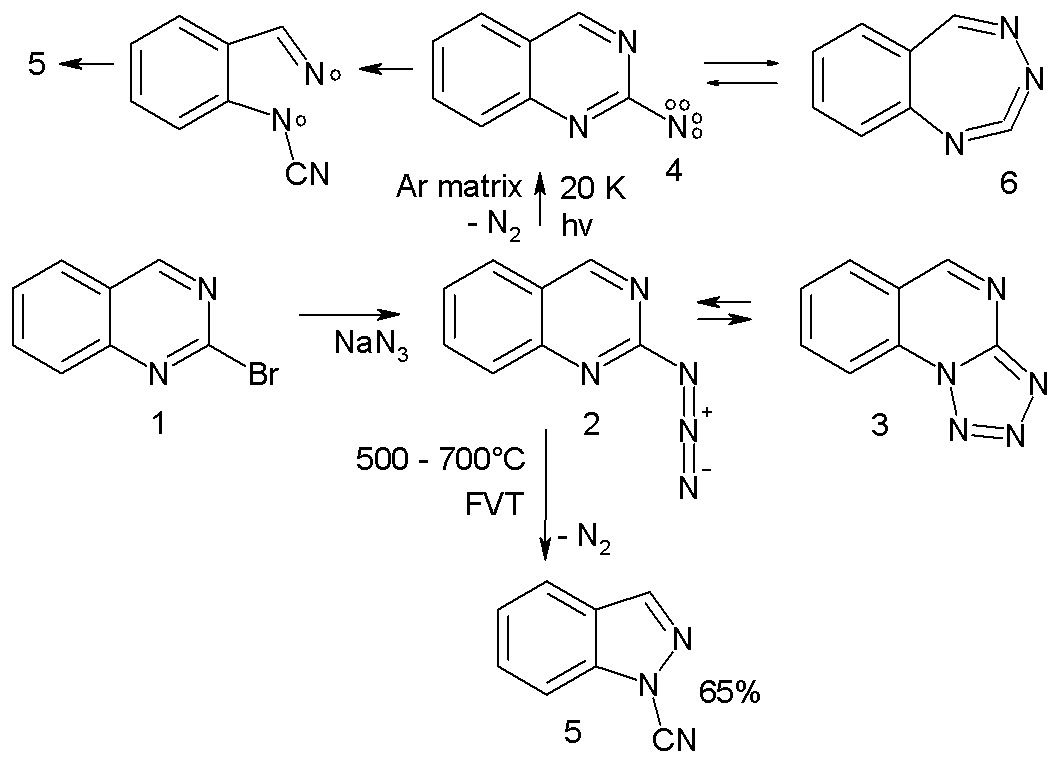 :The nitrene ultimately converts to the ring-opened
:The nitrene ultimately converts to the ring-opened
 In this system one of the nitrogen unpaired electrons is delocalized in the aromatic ring making the compound a σ–σ–π triradical. A
In this system one of the nitrogen unpaired electrons is delocalized in the aromatic ring making the compound a σ–σ–π triradical. A
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, a nitrene or imene () is the nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
analogue of a carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
. The nitrogen atom is uncharged and monovalent, so it has only 6 electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s in its valence level—two covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
ed and four non-bonded electrons. It is therefore considered an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
due to the unsatisfied octet. A nitrene is a reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
and is involved in many chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
s. The simplest nitrene, HN, is called imidogen
Imidogen is an inorganic compound with the chemical formula NH. Like other simple radicals, it is highly reactive and consequently short-lived except as a dilute gas. Its behavior depends on its spin multiplicity.
Production and properties
Imido ...
, and that term is sometimes used as a synonym for the nitrene class.
Electron configuration
In the simplest case, the linear N–H molecule (imidogen) has its nitrogen atomsp hybridized
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to f ...
, with two of its four non-bonded electrons as a lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
in an sp orbital and the other two occupying a degenerate pair of p orbitals
In quantum mechanics, an atomic orbital () is a Function (mathematics), function describing the location and Matter wave, wave-like behavior of an electron in an atom. This function describes an electron's Charge density, charge distribution a ...
. The electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
is consistent with Hund's rule: the low energy form is a triplet with one electron in each of the p orbitals and the high energy form is the singlet with an electron pair filling one p orbital and the other p orbital vacant.
As with carbenes, a strong correlation exists between the spin density
Electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typical ...
on the nitrogen atom which can be calculated in silico
In biology and other experimental sciences, an ''in silico'' experiment is one performed on a computer or via computer simulation software. The phrase is pseudo-Latin for 'in silicon' (correct ), referring to silicon in computer chips. It was c ...
and the zero-field splitting parameter ''D'' which can be derived experimentally from electron spin resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spin ...
. Small nitrenes such as NH or CF3N have D values around 1.8 cm−1 with spin densities close to a maximum value of 2. At the lower end of the scale are molecules with low ''D'' (< 0.4) values and spin density of 1.2 to 1.4 such as 9-anthrylnitrene and 9-phenanthrylnitrene.
Formation
Because nitrenes are so reactive, they are rarely isolated. Instead, they are formed as reactive intermediates during a reaction. There are two common ways to generate nitrenes: * Fromazide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
s by thermolysis
Thermal decomposition, or thermolysis, is a chemical decomposition of a substance caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic ...
or photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons wi ...
, with expulsion of nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
gas. This method is analogous to the formation of carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
s from diazo compound
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds ...
s.
* From isocyanate
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyan ...
s, with expulsion of carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
. This method is analogous to the formation of carbenes from ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The na ...
s.
Since formation of the nitrene typically starts from a diamagnetic precursor, the direct chemical product is a singlet nitrene, which then relaxes to its ground state triplet state. As has been shown for phenylazide as a model system, the direct photoproduct of photochemical-induced N2 loss can either be the singlet or triplet nitrene. By using a triplet sensitizer, the triplet nitrene can also be formed without initial formation of the singlet nitrene.
Isolated Nitrenes
Although highly reactive, some nitrenes could be isolated and characterized recently. In 2019, a triplet nitrene was isolated by Betley and Lancaster, stabilized by coordination to a copper center in a bulky ligand. Later on, Schneider and coworkers characterized Pd and Pt triplet metallonitrenes, where the organic residue is replaced by a metal. In 2024, the groups of Beckmann, Ye and Tan reported the isolation and characterization of organic triplet nitrenes, which are protected from chemical reactivity by an extremely bulky ligand.Reactions
Nitrene reactions include: * Nitrene C–H insertion. A nitrene can easily insert into a carbon to hydrogencovalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
yielding an amine or amide. A singlet nitrene reacts with retention of configuration
Walden inversion is the inversion of a stereogenic center in a chiral molecule in a chemical reaction. Since a molecule can form two enantiomers around a stereogenic center, the Walden inversion converts the configuration of the molecule from ...
. In one study a nitrene, formed by oxidation of a carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general Chemical formula, formula and Chemical structure, structure , which are formally Derivative (chemistry), derived from carbamic acid (). The term includes orga ...
with potassium persulfate
Potassium persulfate is the inorganic compound with the formula K2 S2O8. Also known as potassium peroxydisulfate, it is a white solid that is sparingly soluble in cold water, but dissolves better in warm water. This salt is a powerful oxidant, co ...
, gives an insertion reaction
An insertion reaction is a chemical reaction where one chemical entity (a molecule or molecular fragment) interposes itself into an existing Chemical bond, bond of typically a second chemical entity ''e.g.'':
: + \longrightarrow
The term only ...
into the palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
to nitrogen bond of the reaction product of palladium(II) acetate
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), wh ...
with 2-phenylpyridine to methyl ''N''-(2-pyridylphenyl)carbamate in a cascade reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the p ...
:
:: :A nitrene intermediate is suspected in this C–H insertion involving an
:A nitrene intermediate is suspected in this C–H insertion involving an oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional g ...
, acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of c ...
leading to an isoindole
In organic chemistry and heterocyclic chemistry, isoindole consists of a benzene ring fused with pyrrole. The compound is an isomer of indole. Its reduced form is isoindoline. The parent isoindole is a rarely encountered in the technical lit ...
:
:: * Nitrene cycloaddition. With
* Nitrene cycloaddition. With alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s, nitrenes react to form aziridines
220 px, chemotherapy.html" ;"title="Mitomycin C, an aziridine, is used as a chemotherapy">chemotherapeutic agent by virtue of its antitumour activity.
In organic chemistry, aziridines are organic compounds containing the aziridine functional gr ...
, very often with nitrenoid precursors such as nosyl- or tosyl-substituted 'N''-(phenylsulfonyl)iminohenyliodinane (PhI=NNs or PhI=NTs respectively)) but the reaction is known to work directly with the sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this gro ...
in presence of a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
based catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
such as copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
, palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
, or gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
:
:: :In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
::
:In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
:: :Nitrene transfer takes place next:
::
:Nitrene transfer takes place next:
:: :In this particular reaction both the '' cis''-
:In this particular reaction both the '' cis''-stilbene Stilbene may refer to one of the two stereoisomers of 1,2-diphenylethene:
* (''E'')-Stilbene (''trans'' isomer)
* (''Z'')-Stilbene (''cis'' isomer)
See also
* Stilbenoid
Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C ...
illustrated and the ''trans'' form (not depicted) result in the same ''trans''-aziridine product, suggesting a two-step reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
. The energy difference between triplet and singlet nitrenes can be very small in some cases, allowing interconversion at room temperature. Triplet nitrenes are thermodynamically more stable but react stepwise allowing free rotation and thus producing a mixture of stereochemistry.
* Arylnitrene ring-expansion and ring-contraction: Aryl nitrenes show ring expansion to 7-membered ring cumulene
A cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumu ...
s, ring opening reactions and nitrile formations many times in complex reaction paths. For instance the azide 2 in the scheme below trapped in an argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
matrix
Matrix (: matrices or matrixes) or MATRIX may refer to:
Science and mathematics
* Matrix (mathematics), a rectangular array of numbers, symbols or expressions
* Matrix (logic), part of a formula in prenex normal form
* Matrix (biology), the m ...
at 20 K on photolysis expels nitrogen to the triplet nitrene 4 (observed experimentally with ESR and ultraviolet-visible spectroscopy) which is in equilibrium with the ring-expansion product 6.
: :The nitrene ultimately converts to the ring-opened
:The nitrene ultimately converts to the ring-opened nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The name of the compound is composed of a base, which includes the carbon of the , suffixed with "nitrile", so for example is called " propionitrile" (or pr ...
5 through the diradical
In chemistry, a diradical is a chemical species, molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate energy level, degenerate. The term "diradical" is mainly used to describe organic compounds, where most ...
intermediate 7. In a high-temperature reaction, FVT at 500–600 °C also yields the nitrile 5 in 65% yield. Arylnitrene internalization in combination with carbon deletion strategies have been used for aromatic carbon-nitrogen swap to generate pyridines
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weakly alkaline, water-miscible liquid with a ...
from phenyl azides.
Nitreno radicals
For several compounds containing both a nitrene group and afree radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
group an ESR high-spin quartet has been recorded (matrix, cryogenic temperatures). One of these has an amine oxide
In chemistry, an amine oxide, also known as an amine ''N''-oxide or simply ''N''-oxide, is a chemical compound that has the chemical formula . It contains a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substitue ...
radical group incorporated, another system has a carbon radical group.
:carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
nitrogen radical (imidyl radical) resonance structure
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
makes a contribution to the total electronic picture.
References
{{Functional group Reactive intermediates Free radicals Octet-deficient functional groups Nitrogen compounds