Neutron Absorption on:
[Wikipedia]
[Google]
[Amazon]
Neutron capture is a
 At small
At small
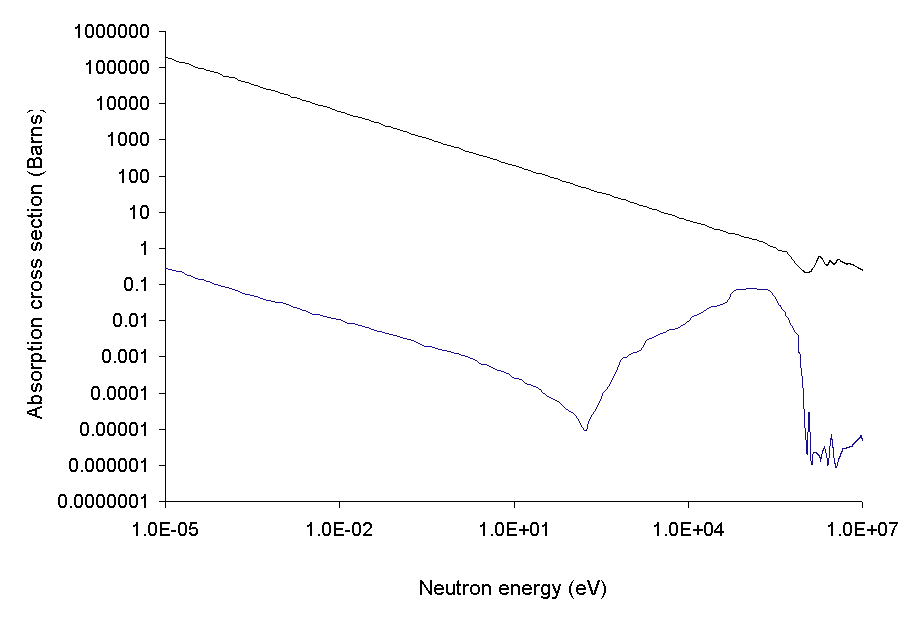 In engineering, the most important neutron absorber is 10 B, used as
In engineering, the most important neutron absorber is 10 B, used as
XSPlot an online neutron cross section plotter
at the International Atomic Energy Agency {{Authority control Nuclear physics Capture Neutron-related techniques
nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
in which an atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the Department_of_Physics_and_Astronomy,_University_of_Manchester , University of Manchester ...
and one or more neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s, which are repelled electrostatically.
Neutron capture plays a significant role in the cosmic nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
of heavy elements. In stars it can proceed in two ways: as a rapid process (r-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for nucleosynthesis, the creation of approximately half of the Atomic nucleus, atomic nuclei Heavy meta ...
) or a slow process (s-process
The slow neutron-capture process, or ''s''-process, is a series of nuclear reactions, reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynt ...
). Nuclei of masses greater than 56 cannot be formed by exothermic thermonuclear reactions (i.e., by nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
) but can be formed by neutron capture.
Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares
A solar flare is a relatively intense, localized emission of electromagnetic radiation in the Stellar atmosphere, Sun's atmosphere. Flares occur in active regions and are often, but not always, accompanied by coronal mass ejections, solar partic ...
.
Neutron capture at small neutron flux
neutron flux
The neutron flux is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total distance travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travelling ...
, as in a nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
, a single neutron is captured by a nucleus. For example, when natural gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
(197Au) is irradiated by neutrons (n), the isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
198Au is formed in a highly excited state, and quickly decays to the ground state of 198Au by the emission of gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
s (). In this process, the mass number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is appro ...
increases by one. This is written as a formula in the form , or in short form . If thermal neutrons
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with ...
are used, the process is called thermal capture.
The isotope 198Au is a beta emitter that decays into the mercury isotope 198Hg. In this process, the atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
rises by one.
Neutron capture at high neutron flux
Ther-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for nucleosynthesis, the creation of approximately half of the Atomic nucleus, atomic nuclei Heavy meta ...
happens inside stars if the neutron flux density is so high that the atomic nucleus has no time to decay via beta emission between neutron captures. The mass number therefore rises by a large amount while the atomic number (i.e., the element) stays the same. When further neutron capture is no longer possible, the highly unstable nuclei decay via many β− decays to beta-stable isotopes of higher-numbered elements.
Capture cross section
The absorptionneutron cross section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of ...
of an isotope of a chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
is the effective cross-sectional area that an atom of that isotope presents to absorption and is a measure of the probability of neutron capture. It is usually measured in barns.
Absorption cross section is often highly dependent on neutron energy
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with ...
. In general, the likelihood of absorption is proportional to the time the neutron is in the vicinity of the nucleus. The time spent in the vicinity of the nucleus is inversely proportional to the relative velocity between the neutron and nucleus. Other more specific issues modify this general principle. Two of the most specified measures are the cross section for thermal neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium wit ...
absorption and the resonance integral, which considers the contribution of absorption peaks at certain neutron energies specific to a particular nuclide
Nuclides (or nucleides, from nucleus, also known as nuclear species) are a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by the A ...
, usually above the thermal range, but encountered as neutron moderation
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely m ...
slows the neutron from an original high energy.
The thermal energy of the nucleus also has an effect; as temperatures rise, Doppler broadening
In atomic physics, Doppler broadening is broadening of spectral lines due to the Doppler effect caused by a distribution of velocities of atoms or molecules. Different velocities of the emitting (or absorbing) particles result in different Doppl ...
increases the chance of catching a resonance peak. In particular, the increase in uranium-238
Uranium-238 ( or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it i ...
's ability to absorb neutrons at higher temperatures (and to do so without fissioning) is a negative feedback
Feedback occurs when outputs of a system are routed back as inputs as part of a chain of cause and effect that forms a circuit or loop. The system can then be said to ''feed back'' into itself. The notion of cause-and-effect has to be handle ...
mechanism that helps keep nuclear reactors under control.
Thermochemical significance
Neutron capture is involved in the formation of isotopes of chemical elements. The energy of neutron capture thus intervenes in thestandard enthalpy of formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, w ...
of isotopes.
Uses
Neutron activation analysis
Neutron activation analysis (NAA) is a nuclear reaction, nuclear process used for determining the concentrations of chemical element, elements in many materials. NAA allows discrete Sampling (statistics), sampling of elements as it disregards the ...
can be used to remotely detect the chemical composition of materials. This is because different elements release different characteristic radiation when they absorb neutrons. This makes it useful in many fields related to mineral exploration and security.
Neutron absorbers
 In engineering, the most important neutron absorber is 10 B, used as
In engineering, the most important neutron absorber is 10 B, used as boron carbide
Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders,
as well as numerous industrial applications. With a Vickers har ...
in nuclear reactor control rods or as boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white ...
as a coolant water additive in pressurized water reactor
A pressurized water reactor (PWR) is a type of light-water nuclear reactor. PWRs constitute the large majority of the world's nuclear power plants (with notable exceptions being the UK, Japan, India and Canada).
In a PWR, water is used both as ...
s. Other neutron absorbers used in nuclear reactors are xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
, cadmium
Cadmium is a chemical element; it has chemical symbol, symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Like z ...
, hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
, gadolinium
Gadolinium is a chemical element; it has Symbol (chemistry), symbol Gd and atomic number 64. It is a silvery-white metal when oxidation is removed. Gadolinium is a malleable and ductile rare-earth element. It reacts with atmospheric oxygen or moi ...
, cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
, samarium, titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
, dysprosium
Dysprosium is a chemical element; it has symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanides, it ...
, erbium
Erbium is a chemical element; it has Symbol (chemistry), symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare- ...
, europium
Europium is a chemical element; it has symbol Eu and atomic number 63. It is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. Europium is the most chemically reactive, least dense, and soft ...
, molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
and ytterbium
Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthani ...
. All of these occur in nature as mixtures of various isotopes, some of which are excellent neutron absorbers. They may occur in compounds such as molybdenum boride, hafnium diboride, titanium diboride, dysprosium titanate and gadolinium titanate.
Hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
absorbs neutrons avidly and it can be used in reactor control rods. However, it is found in the same ores as zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
, which shares the same outer electron shell configuration and thus has similar chemical properties. Their nuclear properties are profoundly different: hafnium absorbs neutrons 600 times better than zirconium. The latter, being essentially transparent to neutrons, is prized for internal reactor parts, including the metallic cladding of the fuel rod
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other nuclear devices to generate energy.
Oxide fuel
For fission reactors, the fuel (typically based on uranium) is usually based o ...
s. To use these elements in their respective applications, the zirconium must be separated from the naturally co-occurring hafnium. This can be accomplished economically with ion-exchange resin
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange, that is also known as an ionex. It is an insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radiu ...
s.
See also
*Beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
* Induced radioactivity
*List of particles
This is a list of known and hypothesized microscopic particles in particle physics, condensed matter physics and cosmology.
Standard Model elementary particles
Elementary particles are particles with no measurable internal structure; that is, ...
*Neutron emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a Atomic nucleus, nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photodisin ...
*Radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
*Rays: α — β — γ — δ
*p-process
The term p-process (''p'' for proton) is used in two ways in the scientific literature concerning the astrophysical origin of the elements (nucleosynthesis). Originally it referred to a proton capture process which was proposed to be the source ...
(proton capture)
References
External links
XSPlot an online neutron cross section plotter
at the International Atomic Energy Agency {{Authority control Nuclear physics Capture Neutron-related techniques