NAPQI on:
[Wikipedia]
[Google]
[Amazon]
NAPQI, also known as NAPBQI or ''N''-acetyl-''p''-benzoquinone imine, is a toxic byproduct produced during the
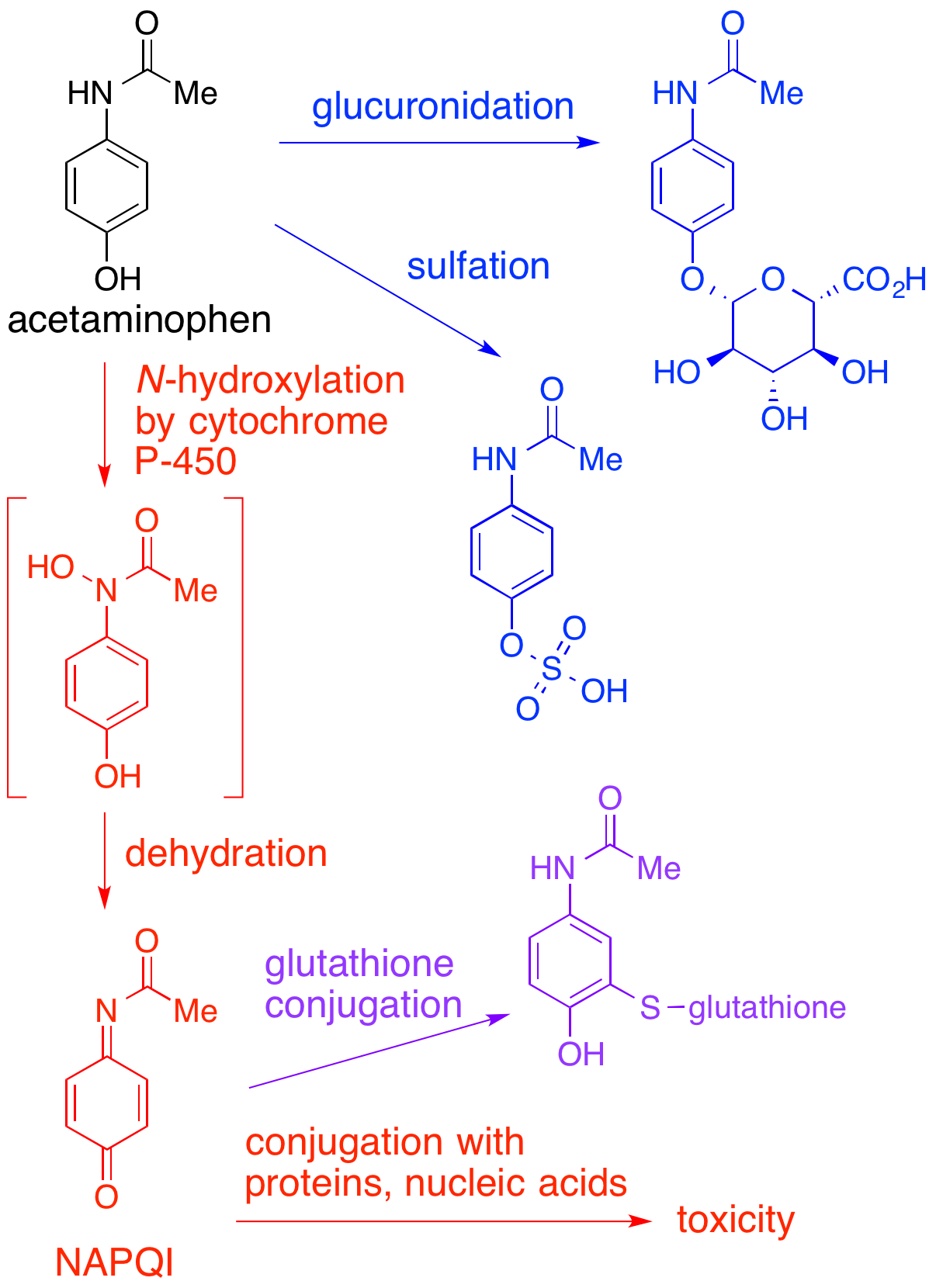 In adults, the primary metabolic pathway for paracetamol is
In adults, the primary metabolic pathway for paracetamol is
xenobiotic metabolism
Xenobiotic metabolism (from the Greek xenos (Greek), xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal bi ...
of the analgesic
An analgesic drug, also called simply an analgesic, antalgic, pain reliever, or painkiller, is any member of the group of drugs used for pain management. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in s ...
paracetamol
Paracetamol, or acetaminophen, is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely available over-the-counter drug sold under various brand names, including Tylenol and Panadol.
Parac ...
(acetaminophen). It is normally produced only in small amounts, and then almost immediately detoxified in the liver.
However, under some conditions in which NAPQI is not effectively detoxified (usually in the case of paracetamol overdose), it causes severe damage to the liver. This becomes apparent 3–4 days after ingestion and may result in death from fulminant liver failure several days after the overdose.
Metabolism
 In adults, the primary metabolic pathway for paracetamol is
In adults, the primary metabolic pathway for paracetamol is glucuronidation
Glucuronidation is often involved in drug metabolism of substances such as drugs, pollutants, bilirubin, androgens, estrogens, mineralocorticoids, glucocorticoids, fatty acid derivatives, retinoids, and bile acids. These linkages involve gly ...
. This yields a relatively non-toxic metabolite, which is excreted into bile and passed out of the body. A small amount of the drug is metabolized via the cytochrome P-450 pathway (to be specific, CYP3A4
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by ''CYP3A4'' gene. It organic redox reaction, oxidizes small foreign organic molecules ( ...
and CYP2E1
Cytochrome P450 2E1 (abbreviated CYP2E1, ) is a member of the cytochrome P450 mixed-function oxidase system, which is involved in the metabolism of xenobiotics in the body. This class of enzymes is divided up into a number of subcategories, inclu ...
) into NAPQI, which is extremely toxic to liver tissue, as well as being a strong biochemical oxidizer. In an average adult, only a small amount (approximately 10% of a therapeutic paracetamol dose) of NAPQI is produced, which is inactivated by conjugation with glutathione
Glutathione (GSH, ) is an organic compound with the chemical formula . It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources ...
(GSH). The amount of NAPQI produced differs in certain populations.
The minimum dosage at which paracetamol causes toxicity usually is 7.5 to 10g in the average person. The lethal dose is usually between 10 g and 15 g. Concurrent alcohol intake lowers these thresholds significantly. Chronic alcoholics may be more susceptible to adverse effects due to reduced glutathione levels. Other populations may experience effects at lower or higher dosages depending on differences in P-450 enzyme activity and other factors which affect the amount of NAPQI produced. In general, however, the primary concern is accidental or intentional paracetamol overdose.
When a toxic dose of paracetamol is ingested, the normal glucuronide pathway is saturated and large amounts of NAPQI are produced. Liver reserves of glutathione are depleted by conjugation with this excess NAPQI. The mechanism by which toxicity results is complex, but is believed to involve reaction between unconjugated NAPQI and critical proteins as well as increased susceptibility to oxidative stress
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal ...
caused by the depletion of glutathione.
Poisoning
The prognosis is good for paracetamol overdoses if treatment is initiated up to 8 hours after the drug has been taken. Most hospitals stock the antidote ( acetylcysteine), which replenishes the liver's supply ofglutathione
Glutathione (GSH, ) is an organic compound with the chemical formula . It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources ...
, allowing the NAPQI to be metabolized safely. Without early administration of the antidote, fulminant liver failure follows, often in combination with kidney failure, and death generally occurs within several days.
Mechanism and antidote
NAPQI becomes toxic when GSH is depleted by an overdose of acetaminophen, Glutathione is an essential antidote to overdose. Glutathione conjugates to NAPQI and helps to detoxify it. In this capacity, it protects cellular protein thiol groups, which would otherwise become covalently modified; when all GSH has been spent, NAPQI begins to bind to certain enzymes like N-10 formyltetrahydrofolate dehydrogenase and glutamate dehydrogenase, reducing their activity and killing the cells in the process. This, along with the depletion of GSH which significantly impairs the function of mitochondria, plays a significant role in the development of paracetamol toxicity. The preferred treatment for an overdose of this painkiller is the administration of ''N''-acetyl-L-cysteine (either via oral or IV administration)), which is processed by cells to L-cysteine and used in the ''de novo'' synthesis of GSH.See also
*Cytochrome P450 oxidase
Cytochromes P450 (P450s or CYPs) are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in ''Escherich ...
*Liver failure
Liver failure is the inability of the liver to perform its normal synthetic and metabolic functions as part of normal physiology. Two forms are recognised, acute and chronic (cirrhosis). Recently, a third form of liver failure known as acute- ...
* Centrilobular necrosis
References
Further reading
* *{{cite journal , vauthors=van de Straat R, de Vries J, Debets AJ, Vermeulen NP , title=The mechanism of prevention of paracetamol-induced hepatotoxicity by 3,5-dialkyl substitution. The roles of glutathione depletion and oxidative stress , journal=Biochem. Pharmacol. , volume=36 , issue=13 , pages=2065–70 , date=July 1987 , pmid=3606627 , doi=10.1016/0006-2952(87)90132-8 Human drug metabolites Chemical pathology Hepatotoxins Imines Toxins