Mukaiyama Aldol Addition on:
[Wikipedia]
[Google]
[Amazon]
In

 In the cited example the Lewis acid TiCl4 is used. First, the Lewis acid activates the aldehyde component followed by carbon-carbon bond formation between the enol silane and the activated aldehyde.
With the loss of a chlorosilane the compound 1 is built. The desired product, a racemate of 2 and 3, is obtained by aqueous work-up.
In the cited example the Lewis acid TiCl4 is used. First, the Lewis acid activates the aldehyde component followed by carbon-carbon bond formation between the enol silane and the activated aldehyde.
With the loss of a chlorosilane the compound 1 is built. The desired product, a racemate of 2 and 3, is obtained by aqueous work-up.
 The analogous
The analogous 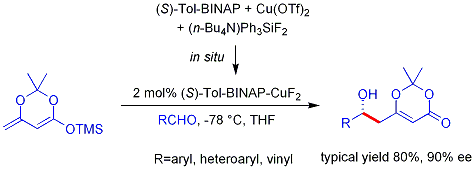
 Ketone reactions of this type require higher reaction temperatures. For this work Mukaiyama was inspired by earlier work done by
Ketone reactions of this type require higher reaction temperatures. For this work Mukaiyama was inspired by earlier work done by  and a second one with an amine chiral ligand and a triflate salt catalyst:
and a second one with an amine chiral ligand and a triflate salt catalyst:
 Utilization of chiral Lewis acid complexes and Lewis bases in asymmetric catalytic processes is the fastest-growing area in the usage of the Mukaiyama aldol reaction.
Utilization of chiral Lewis acid complexes and Lewis bases in asymmetric catalytic processes is the fastest-growing area in the usage of the Mukaiyama aldol reaction.
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the Mukaiyama aldol addition is an organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
and a type of aldol reaction
The aldol reaction (aldol addition) is a Chemical reaction, reaction in organic chemistry that combines two Carbonyl group, carbonyl compounds (e.g. aldehydes or ketones) to form a new β-hydroxy carbonyl compound. Its simplest form might invol ...
between a silyl enol ether
In organosilicon chemistry, silyl enol ethers are a class of organic compounds that share the common functional group , composed of an enolate () bonded to a silane () through its oxygen end and an ethene group () as its carbon end. They are i ...
() and an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
() or formate
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.
Fundamentals
When dissolved in water, formic acid co ...
(). The reaction was discovered by Teruaki Mukaiyama in 1973. His choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
(), or a different aldehyde without self-condensation
In organic chemistry, self-condensation is an organic reaction in which a chemical compound containing a carbonyl group () acts both as the electrophile and the nucleophile in an aldol condensation. It is also called a symmetrical aldol condensat ...
of the aldehyde. For this reason the reaction is used extensively in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
.
General reaction scheme
The Mukaiyama aldol addition is aLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
-mediated addition
Addition (usually signified by the Plus and minus signs#Plus sign, plus symbol, +) is one of the four basic Operation (mathematics), operations of arithmetic, the other three being subtraction, multiplication, and Division (mathematics), divis ...
of enol silanes to carbonyl () compounds. In this reaction, compounds with various organic groups can be used (see educts).
A basic version (R2 = H) without the presence of chiral catalysts
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
is shown below.
A racemic mix of enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...
s is built. If Z- or E-enol silanes are used in this reaction a mixture of four products occurs, yielding two racemates.
Whether the ''anti''-diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
or the ''syn''-diastereomer is built depends largely on reaction conditions, substrates and Lewis acids.
The archetypical reaction is that of the silyl enol ether of cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of ...
, , with benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-li ...
, . At room temperature
Room temperature, colloquially, denotes the range of air temperatures most people find comfortable indoors while dressed in typical clothing. Comfortable temperatures can be extended beyond this range depending on humidity, air circulation, and ...
it produces a diastereomeric mixture of threo (63%) and erythro (19%) β-hydroxyketone
In organic chemistry, a hydroxy ketone (often referred to simply as a ketol) is a functional group consisting of a ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-c ...
as well as 6% of the exocyclic
In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached.
Cyc ...
, enone condensation product. In its original scope the Lewis acid (titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula . It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. is a volatile liquid. Upon contact with humid air, it forms thick clouds o ...
, ) was used in stoichiometric amounts but truly catalytic systems exist as well. The reaction is also optimized for asymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
.
Mechanism
Below, the reaction mechanism is shown with R2 = H:Stereoselection
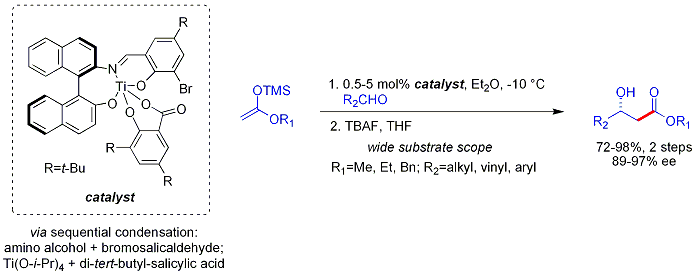
The Mukaiyama aldol reaction does not follow the Zimmerman-Traxler model. Carreira has described particularly useful asymmetric methodology with silyl ketene acetals, noteworthy for its high levels of enantioselectivity and wide substrate scope. The method works on unbranched aliphatic aldehydes, which are often poorelectrophiles
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries ...
for catalytic, asymmetric processes. This may be due to poor electronic and steric differentiation between their enantiofaces.
 The analogous
The analogous vinylogous
In organic chemistry, vinylogy is the transmission of electronic effects through a conjugated organic bonding system. The concept was introduced in 1926 by Ludwig Claisen to explain the acidic properties of formylacetone and related ketoald ...
Mukaiyama aldol process can also be rendered catalytic and asymmetric. The example shown below works efficiently for aromatic (but not aliphatic) aldehydes and the mechanism is believed to involve a chiral, metal-bound dienolate.

Scope
A typical reaction involving two ketones is that betweenacetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Production
Acetophenone is formed as a byproduct of the cumene ...
as the enol and acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
:
Georg Wittig
Georg Wittig (; 16 June 1897 – 26 August 1987) was a German chemist who reported a method for synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize i ...
in 1966 on crossed aldol reactions with lithiated imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s. Competing work with lithium enolate aldol reactions was published also in 1973 by Herbert O. House.
Mukaiyama employed in his rendition of taxol total synthesis (1999) two aldol additions,TBS = t-butyldimethylsilyl, Bn = benzyl, PMB = p-methoxybenzyl ether one with a ketene silyl acetal and excess magnesium bromide
Magnesium bromide are inorganic compounds with the chemical formula , where x can range from 0 to 9. They are all white deliquescent solids. Some magnesium bromides have been found naturally as rare minerals such as: bischofite and carnallite.Gruy ...
:
References
{{reflist Addition reactions Name reactions