Mevacor on:
[Wikipedia]
[Google]
[Amazon]
Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of
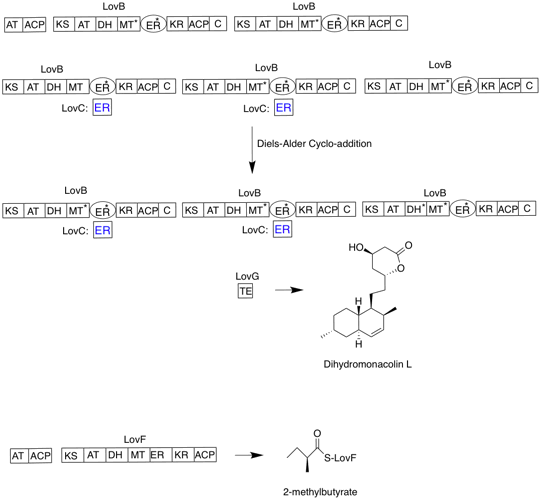 The biosynthesis of lovastatin occurs via an iterative type I polyketide synthase (PKS) pathway. The six genes that encode enzymes that are essential for the biosynthesis of lovastatin are lovB, lovC, lovA, lovD, lovG, and lovF . The synthesis of dihydromonacolin L requires a total of 9-malonyl Coa . It proceeds in the PKS pathway until it reaches (E) a hexaketide, where it undergoes a Diels-Alder cycloaddition to form the fused rings. After cyclization it continues through the PKS pathway until it reaches (I) a nonaketide, which then undergoes release from LovB through the thioesterase encoded by LovG. Dihydromonacolin L, (J), then undergoes oxidation and dehydration via a cytochrome P450 oxygenase encoded by LovA to obtain monacolin J, (L).
The MT domain from lovB is active in the conversion of (B) to (C) when it transfers a methyl group from S-adenosyl-L-methionine (SAM) to the tetraketide (C) . Because LovB contains an inactive ER domain, LovC is required at specific steps to obtain fully reduced products. The domain organization of LovB, LovC, LovG and LovF is shown in Figure 2. The inactive ER domain of lovB is shown with an oval and where LovC acts in trans to LovB is shown with a red box.
In a parallel pathway, the diketide side chain of lovastatin is synthesized by another highly reducing type I polyketide synthase enzyme encoded by LovF . Lastly, the side chain, 2-methylbutyrate (M) is covalently attached to C-8 hydroxy group of monacolin J (L) by a transesterase encoded by LovD to form lovastatin.
The biosynthesis of lovastatin occurs via an iterative type I polyketide synthase (PKS) pathway. The six genes that encode enzymes that are essential for the biosynthesis of lovastatin are lovB, lovC, lovA, lovD, lovG, and lovF . The synthesis of dihydromonacolin L requires a total of 9-malonyl Coa . It proceeds in the PKS pathway until it reaches (E) a hexaketide, where it undergoes a Diels-Alder cycloaddition to form the fused rings. After cyclization it continues through the PKS pathway until it reaches (I) a nonaketide, which then undergoes release from LovB through the thioesterase encoded by LovG. Dihydromonacolin L, (J), then undergoes oxidation and dehydration via a cytochrome P450 oxygenase encoded by LovA to obtain monacolin J, (L).
The MT domain from lovB is active in the conversion of (B) to (C) when it transfers a methyl group from S-adenosyl-L-methionine (SAM) to the tetraketide (C) . Because LovB contains an inactive ER domain, LovC is required at specific steps to obtain fully reduced products. The domain organization of LovB, LovC, LovG and LovF is shown in Figure 2. The inactive ER domain of lovB is shown with an oval and where LovC acts in trans to LovB is shown with a red box.
In a parallel pathway, the diketide side chain of lovastatin is synthesized by another highly reducing type I polyketide synthase enzyme encoded by LovF . Lastly, the side chain, 2-methylbutyrate (M) is covalently attached to C-8 hydroxy group of monacolin J (L) by a transesterase encoded by LovD to form lovastatin.
Image:Cholesterolbiosynthesis.png, Cholesterol biosynthetic pathway
Image:Hmg-reductase.png, HMG CoA reductase reaction
Image:Biosynthesis-dielsalder.png, Biosynthesis using Diels-Alder catalyzed cyclization
Image:Biosynthesis-lovd.png, Biosynthesis using broadly specific acyltransferase
Image:Totalsynthesis1.png, Synthesis of compounds 1 and 2
Image:Totalsynthesis2.png, Complete lovastatin synthesis
cardiovascular disease
Cardiovascular disease (CVD) is any disease involving the heart or blood vessels. CVDs constitute a class of diseases that includes: coronary artery diseases (e.g. angina, heart attack), heart failure, hypertensive heart disease, rheumati ...
. Its use is recommended together with lifestyle changes. It is taken by mouth.
Common side effects include diarrhea, constipation, headache, muscles pains, rash, and trouble sleeping. Serious side effects may include liver problems
Liver disease, or hepatic disease, is any of many diseases of the liver. If long-lasting it is termed chronic liver disease. Although the diseases differ in detail, liver diseases often have features in common.
Liver diseases
File:Ground gla ...
, muscle breakdown, and kidney failure
Kidney failure, also known as renal failure or end-stage renal disease (ESRD), is a medical condition in which the kidneys can no longer adequately filter waste products from the blood, functioning at less than 15% of normal levels. Kidney fa ...
. Use during pregnancy
Pregnancy is the time during which one or more offspring gestation, gestates inside a woman's uterus. A multiple birth, multiple pregnancy involves more than one offspring, such as with twins.
Conception (biology), Conception usually occurs ...
may harm the baby and use during breastfeeding
Breastfeeding, also known as nursing, is the process where breast milk is fed to a child. Infants may suck the milk directly from the breast, or milk may be extracted with a Breast pump, pump and then fed to the infant. The World Health Orga ...
is not recommended. It works by decreasing the liver's ability to produce cholesterol by blocking the enzyme HMG-CoA reductase.
Lovastatin was patented in 1979 and approved for medical use in 1987. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active ch ...
. In 2022, it was the 111th most commonly prescribed medication in the United States, with more than 5million prescriptions.
Medical uses
The primary uses of lovastatin is for the treatment ofdyslipidemia
Dyslipidemia is a metabolic disorder characterized by abnormally high or low amounts of any or all lipids (e.g. fats, triglycerides, cholesterol, phospholipids) or lipoproteins in the blood. Dyslipidemia is a risk factor for the development of ...
and the prevention of cardiovascular disease
Cardiovascular disease (CVD) is any disease involving the heart or blood vessels. CVDs constitute a class of diseases that includes: coronary artery diseases (e.g. angina, heart attack), heart failure, hypertensive heart disease, rheumati ...
. It is recommended to be used only after other measures, such as diet, exercise, and weight reduction, have not improved cholesterol levels.
Side effects
Lovastatin is usually well tolerated, with the most common side effects being, in approximately descending order of frequency: creatine phosphokinase elevation,flatulence
Flatulence is the expulsion of gas from the Gastrointestinal tract, intestines via the anus, commonly referred to as farting. "Flatus" is the medical word for gas generated in the stomach or bowels. A proportion of intestinal gas may be swal ...
, abdominal pain, constipation, diarrhoea
Diarrhea (American English), also spelled diarrhoea or diarrhœa (British English), is the condition of having at least three loose, liquid, or watery bowel movements in a day. It often lasts for a few days and can result in dehydration d ...
, muscle aches or pains, nausea, indigestion, weakness, blurred vision, rash, dizziness and muscle cramps. As with all statin drugs, it can occasionally cause myopathy
In medicine, myopathy is a disease of the muscle in which the muscle fibers do not function properly. ''Myopathy'' means muscle disease ( Greek : myo- ''muscle'' + patheia '' -pathy'' : ''suffering''). This meaning implies that the primary defec ...
, hepatotoxicity
Hepatotoxicity (from ''hepatic toxicity'') implies chemical-driven liver damage. Drug-induced liver injury (DILI) is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdr ...
(liver damage), dermatomyositis
Dermatomyositis (DM) is a Chronic condition, long-term inflammatory disorder, inflammatory Autoimmune disease, autoimmune disorder which affects the skin and the muscles. Its symptoms are generally a skin rash and worsening muscle weakness over ...
or rhabdomyolysis. This can be life-threatening if not recognised and treated in time, so any unexplained muscle pain or weakness whilst on lovastatin should be promptly mentioned to the prescribing doctor. Other uncommon side effects that should be promptly mentioned to either the prescribing doctor or an emergency medical service include:
These less serious side effects should still be reported if they persist or increase in severity:
Contraindications
Contraindication
In medicine, a contraindication is a condition (a situation or factor) that serves as a reason not to take a certain medical treatment due to the harm that it would cause the patient. Contraindication is the opposite of indication, which is a rea ...
s, conditions that warrant withholding treatment with lovastatin, include pregnancy, breast feeding, and liver disease. Lovastatin is contraindicated during pregnancy (Pregnancy Category X); it may cause birth defects such as skeletal deformities or learning disabilities. Owing to its potential to disrupt infant lipid metabolism, lovastatin should not be taken while breastfeeding. Patients with liver disease should not take lovastatin.
Interactions
As withatorvastatin
Atorvastatin, sold under the brand name Lipitor among others, is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a ...
, simvastatin
Simvastatin, sold under the brand name Zocor among others, is a statin, a type of lipid-lowering medication. It is used along with exercise, diet, and weight loss to decrease hyperlipidemia, elevated lipid levels. It is also used to decrease t ...
, and other statin drugs metabolized via CYP3A4
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by ''CYP3A4'' gene. It organic redox reaction, oxidizes small foreign organic molecules ( ...
, drinking grapefruit
The grapefruit (''Citrus'' × ''paradisi'') is a subtropical citrus tree known for its relatively large, sour to semi-sweet, somewhat bitter fruit. The flesh of the fruit is segmented and varies in color from pale yellow to dark red.
Grapefru ...
juice during lovastatin therapy may increase the risk of side effects. Components of grapefruit juice, the flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.
Chemically, flavonoids ...
naringin, or the furanocoumarin bergamottin inhibit CYP3A4 ''in vitro'', and may account for the ''in vivo'' effect of grapefruit juice concentrate decreasing the metabolic clearance of lovastatin, and increasing its plasma concentrations.
Mechanism of action
Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme that catalyzes the conversion of HMG-CoA to mevalonate. Mevalonate is a required building block for cholesterol biosynthesis and lovastatin interferes with its production by acting as a reversible competitive inhibitor for HMG-CoA, which binds to the HMG-CoA reductase. Lovastatin is aprodrug
A prodrug is a pharmacologically inactive medication or compound that, after intake, is metabolized (i.e., converted within the body) into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be ...
, an inactive lactone in its native form, the gamma-lactone closed ring form in which it is administered, is hydrolysed in vivo to the β-hydroxy acid open ring form; which is the active form.
Lovastatin and other statins have been studied for their chemopreventive and chemotherapeutic effects. No such effects were seen in the early studies. More recent investigations revealed some chemopreventive and therapeutic effects, for certain types of cancer, especially in combination of statins with other anticancer drugs. It is likely that these effect are mediated by the properties of statins to reduce proteasome activity, leading to an accumulation of cyclin-dependent kinase
Cyclin-dependent kinases (CDKs) are a predominant group of serine/threonine protein kinases involved in the regulation of the cell cycle and its progression, ensuring the integrity and functionality of cellular machinery. These regulatory enzym ...
inhibitors p21 and p27, and to subsequent G1-phase arrest, as seen in cells of different cancer lines.
History
Compactin and lovastatin, natural products with a powerful inhibitory effect on HMG-CoA reductase, were discovered in the 1970s, and taken into clinical development as potential drugs for lowering LDL cholesterol. In 1982, some small-scale clinical investigations of lovastatin, a polyketide-derived natural product isolated from ''Aspergillus terreus'', in very high-risk patients were undertaken, in which dramatic reductions in LDL cholesterol were observed, with very few adverse effects. After the additional animal safety studies with lovastatin revealed no toxicity of the type thought to be associated with compactin, clinical studies continued. Large-scale trials confirmed the effectiveness of lovastatin. Observed tolerability continued to be excellent, and lovastatin was approved by the US FDA in 1987. It was the first statin approved by the FDA. Lovastatin is also naturally produced by certain higherfungi
A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified as one ...
, such as '' Pleurotus ostreatus'' (oyster mushroom) and closely related '' Pleurotus'' spp. Research into the effect of oyster mushroom and its extracts on the cholesterol levels of laboratory animals has been extensive, although the effect has been demonstrated in a very limited number of human subjects.
In 1998, the FDA placed a ban on the sale of dietary supplements derived from red yeast rice, which naturally contains lovastatin, arguing that products containing prescription agents require drug approval. Judge Dale A. Kimball of the United States District Court for the District of Utah, granted a motion by Cholestin's manufacturer, Pharmanex, that the agency's ban was illegal under the 1994 Dietary Supplement Health and Education Act because the product was marketed as a dietary supplement, not a drug.
The objective is to decrease excess levels of cholesterol to an amount consistent with maintenance of normal body function. Cholesterol is biosynthesized in a series of more than 25 separate enzymatic reactions that initially involves three successive condensations of acetyl-CoA units to form the six-carbon compound 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA). This is reduced to mevalonate and then converted in a series of reactions to the isoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. It is produced by many plants and animals (including humans) and its polymers ar ...
s that are building-blocks of squalene
Squalene is an organic compound. It is a triterpene with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as '' Squalus'' is a genus of sharks). ...
, the immediate precursor to sterols, which cyclizes to lanosterol (a methylated sterol) and further metabolized to cholesterol. A number of early attempts to block the synthesis of cholesterol resulted in agents that inhibited late in the biosynthetic pathway between lanosterol and cholesterol. A major rate-limiting step in the pathway is at the level of the microsomal enzyme that catalyzes the conversion of HMG CoA to mevalonic acid, and that has been considered to be a prime target for pharmacologic intervention for several years.
HMG CoA reductase occurs early in the biosynthetic pathway and is among the first committed steps to cholesterol formulation. Inhibition of this enzyme could lead to accumulation of HMG CoA, a water-soluble intermediate that is, then, capable of being readily metabolized to simpler molecules. This inhibition of reductase would lead to accumulation of lipophylic intermediates with a formal sterol ring.
Lovastatin was the first specific inhibitor of HMG CoA reductase to receive approval for the treatment of hypercholesterolemia. The first breakthrough in efforts to find a potent, specific, competitive inhibitor of HMG CoA reductase occurred in 1976, when Endo ''et al.'' reported the discovery of mevastatin, a highly functionalized fungal metabolite, isolated from cultures of ''Penicillium citrium''.
Biosynthesis
 The biosynthesis of lovastatin occurs via an iterative type I polyketide synthase (PKS) pathway. The six genes that encode enzymes that are essential for the biosynthesis of lovastatin are lovB, lovC, lovA, lovD, lovG, and lovF . The synthesis of dihydromonacolin L requires a total of 9-malonyl Coa . It proceeds in the PKS pathway until it reaches (E) a hexaketide, where it undergoes a Diels-Alder cycloaddition to form the fused rings. After cyclization it continues through the PKS pathway until it reaches (I) a nonaketide, which then undergoes release from LovB through the thioesterase encoded by LovG. Dihydromonacolin L, (J), then undergoes oxidation and dehydration via a cytochrome P450 oxygenase encoded by LovA to obtain monacolin J, (L).
The MT domain from lovB is active in the conversion of (B) to (C) when it transfers a methyl group from S-adenosyl-L-methionine (SAM) to the tetraketide (C) . Because LovB contains an inactive ER domain, LovC is required at specific steps to obtain fully reduced products. The domain organization of LovB, LovC, LovG and LovF is shown in Figure 2. The inactive ER domain of lovB is shown with an oval and where LovC acts in trans to LovB is shown with a red box.
In a parallel pathway, the diketide side chain of lovastatin is synthesized by another highly reducing type I polyketide synthase enzyme encoded by LovF . Lastly, the side chain, 2-methylbutyrate (M) is covalently attached to C-8 hydroxy group of monacolin J (L) by a transesterase encoded by LovD to form lovastatin.
The biosynthesis of lovastatin occurs via an iterative type I polyketide synthase (PKS) pathway. The six genes that encode enzymes that are essential for the biosynthesis of lovastatin are lovB, lovC, lovA, lovD, lovG, and lovF . The synthesis of dihydromonacolin L requires a total of 9-malonyl Coa . It proceeds in the PKS pathway until it reaches (E) a hexaketide, where it undergoes a Diels-Alder cycloaddition to form the fused rings. After cyclization it continues through the PKS pathway until it reaches (I) a nonaketide, which then undergoes release from LovB through the thioesterase encoded by LovG. Dihydromonacolin L, (J), then undergoes oxidation and dehydration via a cytochrome P450 oxygenase encoded by LovA to obtain monacolin J, (L).
The MT domain from lovB is active in the conversion of (B) to (C) when it transfers a methyl group from S-adenosyl-L-methionine (SAM) to the tetraketide (C) . Because LovB contains an inactive ER domain, LovC is required at specific steps to obtain fully reduced products. The domain organization of LovB, LovC, LovG and LovF is shown in Figure 2. The inactive ER domain of lovB is shown with an oval and where LovC acts in trans to LovB is shown with a red box.
In a parallel pathway, the diketide side chain of lovastatin is synthesized by another highly reducing type I polyketide synthase enzyme encoded by LovF . Lastly, the side chain, 2-methylbutyrate (M) is covalently attached to C-8 hydroxy group of monacolin J (L) by a transesterase encoded by LovD to form lovastatin.
Total synthesis
A major bulk of work in the synthesis of lovastatin was done by M. Hirama in the 1980s. Hirama synthesized compactin and used one of the intermediates to follow a different path to get to lovastatin. The synthetic sequence is shown in the schemes below. The γ-lactone was synthesized using Yamada methodology starting with glutamic acid. Lactone opening was done using lithium methoxide inmethanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
and then silylation to give a separable mixture of the starting lactone and the silyl ether. The silyl ether on hydrogenolysis followed by Collins oxidation gave the aldehyde. Stereoselective preparation of (E,E)-diene was accomplished by addition of trans-crotyl phenyl sulfone anion, followed by quenching with Ac2O and subsequent reductive elimination of sulfone acetate. Condensation of this with lithium anion of dimethyl methylphosphonate gave compound 1. Compound 2 was synthesized as shown in the scheme in the synthetic procedure. Compounds 1 and 2 were then combined using 1.3 eq sodium hydride in THF followed by reflux in chlorobenzene for 82 hr under nitrogen to get the enone 3.
Simple organic reactions were used to get to lovastatin as shown in the scheme.
Society and culture
Natural sources
Lovastatin is a naturally occurring compound found in low concentrations in food such as oyster mushrooms, red yeast rice, and Pu-erh.Brand names
Mevacor, Advicor (as a combination with niacin), Altocor, AltoprevOther applications
In plant physiology, lovastatin has occasionally been used as inhibitor ofcytokinin
Cytokinins (CK) are a class of plant hormones that promote cell division, or cytokinesis, in plant roots and shoots. They are involved primarily in Cell (biology), cell growth and cellular differentiation, differentiation, but also affect apical ...
biosynthesis.
References
{{Portal bar , Medicine Wikipedia medicine articles ready to translate Statins Carboxylate esters Delta-lactones Total synthesis Bicyclic compounds Drugs developed by Merck & Co. Tetrahydropyrans