metal dithiolene complex on:
[Wikipedia]
[Google]
[Amazon]
Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species along with 1,2-diselenolene derivatives, are unsaturated bidentate

 1,2-Dithiolene complexes applications are numerous, and span from superconductivity, to linear and non linear optics, to biochemistry. Commercial applications of 1,2-dithiolene complexes are limited. A few dithiolene complexes have been commercialized as dyes in laser applications (Q-switching, mode-locking). 1,2-Dithiolene complexes have been discussed in the context of conductivity,
1,2-Dithiolene complexes applications are numerous, and span from superconductivity, to linear and non linear optics, to biochemistry. Commercial applications of 1,2-dithiolene complexes are limited. A few dithiolene complexes have been commercialized as dyes in laser applications (Q-switching, mode-locking). 1,2-Dithiolene complexes have been discussed in the context of conductivity,

ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
wherein the two donor atoms are sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
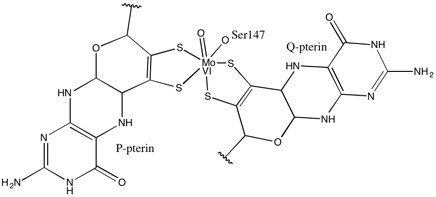
. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.
Dithiolene metal complexes have been studied since the 1960s when they were first popularized by Gerhard N. Schrauzer and Volker P. Mayweg, who prepared nickel bis(stilbene-1,2-dithiolate) () by the reaction of nickel sulfide
Nickel sulfide is any inorganic compound with the formula NixSy. These compounds range in color from bronze (Ni3S2) to black (NiS2). The nickel sulfide with simplest stoichiometry is NiS, also known as the mineral millerite. From the economic ...
and diphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a Transition metal alkyne complex, ligand in ...
. The structural, spectroscopic, and electrochemical properties of many related complexes have been described.
Structure
Dithiolene metal complexes can be found in coordination compounds where the metal centre is coordinated by one, two, or three dithiolene ligands. The tris(dithiolene) complexes were the first examples of trigonal prismatic geometry in coordination chemistry. One example is . Similar structures have been observed for several other metals. Because of the unusual redox and intense optical properties of dithiolenes, the electronic structure of dithiolene complexes has been the subject of intense studies. 1,2-Dithiolene ligands can exist in threeoxidation states
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms are fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Concep ...
: the dianionic "ene-1,2-dithiolate", the neutral "1,2-dithioketone," and a monoanionic radical intermediate between these two. When the latter two are complexed to a metal centre, the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
of the ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
(and therefore the metal centre) cannot be easily defined. Such ligands are therefore referred to as non-innocent. The substituents on the backbone of the dithiolene ligand, R and R', affect the properties of the resulting metal complex in the expected way. Long chains confer solubility in less polar solvents. Electron acceptors (e.g. cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
, acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
) stabilize reduced and anionic complexes. Derivatives are known where the substituents are the same, symmetrical dithiolenes (R = R') are more common than unsymmetrical.
Due to their delocalized electronic structure, 1,2-dithiolene complexes undergo reversible redox reaction. When oxidized, dithiolene complexes have greater 1,2-dithioketone character. In reduced complexes, the ligand assumes more ene-1,2-dithiolate character. These descriptions are evaluated by examination of differences in C-C and C-S bond distances. The true structure lies somewhere between these resonance structures. Reflecting the impossibility to provide an unequivocal description of the structure, McCleverty introduced the term 'dithiolene' to give a general name for the ligand that does not specify a particular oxidation state. This suggestion was generally accepted, and 'dithiolene' is now a universally accepted term. Only more recently the radical nature of monoanionic 1,2-dithiolene ligands has been pointed out. While few examples of authentic dithiolene radicals have been reported, diamagnetism in neutral bis(1,2-dithiolene) complexes of divalent transition metal ions should be considered as a consequence of a string antiferromagnetic coupling between the two radical ligands.
:
Applications and occurrence
1,2-Dithiolene metal complexes occur widely in nature in the form of the molybdopterin-bound Mo and W-containing enzymes. 1,2-Dithiolene complexes applications are numerous, and span from superconductivity, to linear and non linear optics, to biochemistry. Commercial applications of 1,2-dithiolene complexes are limited. A few dithiolene complexes have been commercialized as dyes in laser applications (Q-switching, mode-locking). 1,2-Dithiolene complexes have been discussed in the context of conductivity,
1,2-Dithiolene complexes applications are numerous, and span from superconductivity, to linear and non linear optics, to biochemistry. Commercial applications of 1,2-dithiolene complexes are limited. A few dithiolene complexes have been commercialized as dyes in laser applications (Q-switching, mode-locking). 1,2-Dithiolene complexes have been discussed in the context of conductivity, magnetism
Magnetism is the class of physical attributes that occur through a magnetic field, which allows objects to attract or repel each other. Because both electric currents and magnetic moments of elementary particles give rise to a magnetic field, ...
, and nonlinear optics
Nonlinear optics (NLO) is the branch of optics that describes the behaviour of light in Nonlinearity, nonlinear media, that is, media in which the polarization density P responds non-linearly to the electric field E of the light. The non-linearity ...
. It was proposed to use dithiolene metal complexes that bind unsaturated hydrocarbons at the sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
centers for industrial olefin (alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
) purifications. However, the complexities within such systems became later apparent, and it was argued that more research would be needed before using metal dithiolene complexes in alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
purifications may become practical.
Preparation
From alkenedithiolates
Most dithiolene complexes are prepared by reaction of alkali metal salts of 1,2-alkenedithiolates with metal halides. A thiolate is the conjugate base of athiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
, so alkenedithiolate is, formally speaking, the conjugate base of an alkenedithiol. Common alkenedithiolates are 1,3-dithiole-2-thione-4,5-dithiolate and maleonitriledithiolate ():
:
Some alkenedithiolates are generated in situ, often by complex organic reactions:
:
Once generated, these anions are deployed as ligands:
:
Often the initially formed, electron-rich complex undergoes spontaneous air-oxidation:
:

From acyloins
An early and still powerful method for the synthesis of dithiolenes entails the reaction of α-hydroxyketones, acyloins, with followed by hydrolysis and treatment of the mixture with metal salts. This method is used to prepare (Ar =aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
).
From dithietes
Although 1,2-dithiones are rare and thus not useful precursors, their valence isomer, the 1,2- dithietes are occasionally used. One of the more common dithiete is the distillable . This electrophilic reagent oxidatively adds to many low valent metals to give bis- and tris(dithiolene) complexes. : :By reactions of metal sulfides with alkynes
Species of the type were first prepared by reactions of nickel sulfides withdiphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a Transition metal alkyne complex, ligand in ...
. More modern versions of this method entail the reaction of electrophilic acetylenes such as dimethyl acetylenedicarboxylate with well defined polysulfido complexes.
History and nomenclature
Early studies on dithiolene ligands, although not called by that name until the 1960s, focused on the quinoxaline-2,3-dithiolates and 3,4- toluenedithiolates, which form brightly colored precipitates with several metal centres. Such species were originally of interest in analytical chemistry. Dithiolenes lacking benzene backbones represented an important development of the area, especially maleonitrile-1,2-dithiolate ("mnt"), , and ethylenedithiolene, .References
{{Coordination complexes Coordination complexes Thiols Chelating agents