Metal Carbido Complex on:
[Wikipedia]
[Google]
[Amazon]
A metal carbido complex is a







coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
that contains a carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atom as a ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
. They are analogous to metal nitrido complexes. Carbido complexes are a molecular subclass of carbides
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of t ...
, which are prevalent in organometallic and inorganic chemistry. Carbido complexes represent models for intermediates in Fischer–Tropsch synthesis, olefin metathesis
In organic chemistry, Olefin Metathesis or Alkene Metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the Bond cleavage, scission and regeneration of carbon-carbon double bonds. Because of the ...
, and related catalytic industrial processes. Ruthenium-based carbido complexes are by far the most synthesized and characterized to date. Although, complexes containing chromium, gold, iron, nickel, molybdenum, osmium, rhenium, and tungsten cores are also known. Mixed-metal carbides are also known.
Carbido clusters
Most molecular carbido complexes are clusters, usually featuring carbide as a six-foldbridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...
. Examples include Rh6C(CO)15">Rhodium.html" ;"title="nowiki/> Rh6C(CO)15sup>2−, and [Doubly bridging carbide ligands
Bridging carbido ligands can be subdivided into three classes: *cumulenic , *metallocarbyne , and *polar covalent . Cumulenic compounds generally bridge two metal atoms of the same element and are symmetrical. However, there are exceptions to this. In contrast, metallocarbyne compounds are generally constitutionally heterobimetallic, with complexes containing varying coordination geometries being common. These moieties have been able to serve as precursors to elaborate molecular scaffolds such as porphyrin derivatives. The polar covalent class is distinguished from metallocarbynes by a very fine line. This carbide-metal interaction is considered labile in nature. Carbon here can be understood fundamentally as being similar to CO ligands, that is, dative (L-type). Although, this class has also been described to some extent being analogous to the behavior of Lewis acid adduct-forming terminal nitrido and oxo complexes e.g. (PMe2Ph)2Cl-Re≡N-BCl3 and tBu(CH2)3(Br)W=O-AlBr3.
Terminal carbides
In rare cases, carbido ligands are terminal. One example is with a Ru-C distance of 163 pm, typical for a triple bond. The complex can be obtained by metathesis ofvinyl acetate
Vinyl acetate is an organic compound with the Chemical formula, formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate, ethylene-vinyl acetate copolymers, polyvinyl alcohol, and other important industrial polymers.
Prod ...
to give results in a metastable complex, which eliminates acetic acid.
Such transition metal, one coordinate-carbon bonded complexes are comparable to carbon monoxide, cyanide, and isonitrile analogues. These carbides can be used as synthons to access a wide range of carbyne complexes, the most notable being Fischer carbynes. American chemist Christopher C. Cummins is one of the pioneers of this area.
Preparative routes and characterization
Carbido clusters
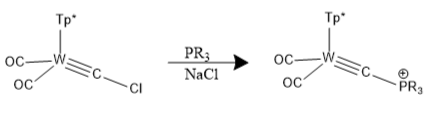
Synthesis of carbido clusters can be accomplished by hydrolysis, thermolysis of labile ligands, thermal rearrangements, and photolysis. Their synthesis has historically been crudely achieved by serendipitous chance following apparent random molecular organization. One example is the following reaction: :
Doubly bridging carbide ligands
Cumulenic
Synthetic routes to cumulenic carbido complexes can be efficient and lead to rapid, near quantitative product formation with simple purifications. This dimerization involves the formation of a vinylidene from an alkyne. Mechanistically, there are various proposed pathways, starting with oxidative addition of the alkyne to the metal core, followed by either intramolecular 1,2-H shifts or intermolecular 1,3-H shifts. For Ruthenium coordination complexes, bridging Ru-Cl bond lengths have been observed to lie in the range of 1.76-1.8 Å. Ru-C bonds can vary significantly as a result of trans effect phenomena which is caused by the respective ethylene and vinylidene ligands.

Metallocarbyne
The appropriate halocarbyne precursors of choice can be reacted with organolithium reagents to afford the respective lithiocarbyne derivate by virtue of lithium/halogen exchange. This species can serve as a lynchpin for subsequent carbide linkage with an additional metal complex. Phosphine-based analogues were first introduced by Templeton and co. These types of complexes can be characterized crystallographically and are distinguishable by their Cs symmetry.

Polar covalent
Addition of tricyclohexylphosphine to the carbene complex (PPh3)2(Cl)2Ru=C(CHCO2Me)2 results in olefin extrusion and yields an air stable anionic carbido complex. This species displaces a dimethyl sulfide ligand from PdCl2(SMe)2 to give the μ-carbido bimetallic complex (PCy3)2Cl2Ru≡C-PdCl2(SMe2). Spark towards a novel type of bonding was proposed following empirical observations wherein the carbido-palladium interaction could be readily disturbed. Reversible coordination ensues upon exposure of the bimetallic complex to carbon monoxide. Additionally, no coordination occurs if the anionic carbido complex contains bulky ligands such as H2IMes. This indicates that the thermodynamic sink towards making the C-M bond is not very favorable, suggesting a weak interaction. Although not intuitive, characterization of this type of bonding can be inferred if 13C NMR shifts are observed to be far downfield, and C-M bond lengths are similar to those of complexes proven to contain carbon-based σ-donor ligands such as Et2H2Im)PdCl(μ-Cl)sub>2.
Terminal carbido ligands
Metathesis using Grubbs-type alkylidene complexes can be used to synthesize terminal carbido-containing complexes. One example is RuC(PCy3)2Cl2 with a Ru-C distance of 163 pm, typical for a triple bond. The complex can be obtained by metathesis ofvinyl acetate
Vinyl acetate is an organic compound with the Chemical formula, formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate, ethylene-vinyl acetate copolymers, polyvinyl alcohol, and other important industrial polymers.
Prod ...
to give u(CH-''p''-C6H4Me)(PCy3)2Cl2results in a metastable Ru(Cl2)(PCy3)2C2HOAc complex, which eliminates acetic acid.
The "naked" carbido ligand is weakly basic, forming complexes with other metal centers. The C-M bond is typically found to be around 1.65 Å. The 13 C NMR resonance values for the carbido carbons vary widely, but range from δ211-406. Another example of a terminal carbido complex is Li oC(NR2)3(Mo-C distance of 172 pm), which forms upon deprotonation of the respective methylidyne precursor.

See also
*Metallocarbohedryne A metallocarbohedryne (met-car) is any one of a family of chemical compounds with the generic molecular formula , where M is a transition metal such as titanium, vanadium, zirconium, niobium, hafnium, molybdenum, chromium, or iron.
These compounds ...
("met-car"), a stable cluster with formula (M = Ti, Zr, V, etc.)
References
{{Coordination complexes Organometallic compounds Organometallic chemistry