Mesoporous Organosilica on:
[Wikipedia]
[Google]
[Amazon]
Mesoporous organosilica (periodic mesoporous organosilicas, PMO) are a type of
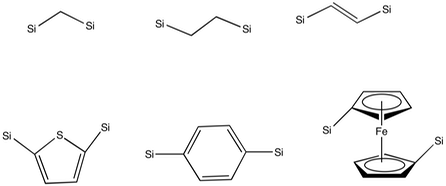

 Anchoring a homogeneous catalyst onto a mesoporous organosilicas framework has two primary disadvantages: the bulky group in the pore can block travel of guest molecules through it, and preparation of candidate molecules for anchoring to the framework is difficult. However, anchoring can create heterogeneous catalysts for a wide variety of chemical transformations:
Anchoring a homogeneous catalyst onto a mesoporous organosilicas framework has two primary disadvantages: the bulky group in the pore can block travel of guest molecules through it, and preparation of candidate molecules for anchoring to the framework is difficult. However, anchoring can create heterogeneous catalysts for a wide variety of chemical transformations:
 Mesoporous organosilicas can be functionalized give adsorbants, for removal specific contaminants from air and water. Candidate adsorbants include toxic heavy metals, radioactive material, and various organic pollutants have been synthesized.
Mesoporous organosilicas have been functionalized with fluorescent probes. The advantage of this material as a sensor is its high surface area combined with the high specificity achievable by careful functionalization. Mesoporous organosilicas have been used to sense a wide variety of analytes: metals, industrial pollutants, small organic molecules, and large biological molecules.
Mesoporous organosilicas have been tested as potential materials for separation using
Mesoporous organosilicas can be functionalized give adsorbants, for removal specific contaminants from air and water. Candidate adsorbants include toxic heavy metals, radioactive material, and various organic pollutants have been synthesized.
Mesoporous organosilicas have been functionalized with fluorescent probes. The advantage of this material as a sensor is its high surface area combined with the high specificity achievable by careful functionalization. Mesoporous organosilicas have been used to sense a wide variety of analytes: metals, industrial pollutants, small organic molecules, and large biological molecules.
Mesoporous organosilicas have been tested as potential materials for separation using
silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
containing organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
groups that give rise to mesoporosity
A mesoporous material (or super nanoporous ) is a Nanoporous materials, nanoporous material containing wiktionary:pore, pores with diameters between 2 and 50 nm, according to International Union of Pure and Applied Chemistry, IUPAC nomenclat ...
. They exhibit pore size ranging from 2 nm - 50 nm, depending on the organic substituents. In contrast, zeolites
Zeolites are a group of several Microporous material, microporous, crystalline aluminosilicate minerals commonly used as commercial adsorption, adsorbents and Catalysis, catalysts. They mainly consist of silicon, aluminium, oxygen, and have the ge ...
exhibit pore sizes less than a nanometer. PMOs have potential applications as catalysts
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
, adsorbents
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fl ...
, trapping agents, drug delivery
Drug delivery involves various methods and technologies designed to transport pharmaceutical compounds to their target sites helping therapeutic effect. It involves principles related to drug preparation, route of administration, site-specif ...
agents, stationary phases in chromatography
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it ...
and chemical sensors
A sensor is often defined as a device that receives and responds to a signal or stimulus. The stimulus is the quantity, property, or condition that is sensed and converted into electrical signal.
In the broadest definition, a sensor is a devi ...
.
History
The breakthrough report in this area described the use of surfactants to produce periodic mesoporous silicas (PMS) in 1992 with pores larger than that of zeolites. Early mesoporous organosilicas developed had organic groups attached terminally to the silica surface. They were prepared either by grafting of organic group onto the channel walls or by template-directed co-condensation. For example, by modifying the channels of PMSs withalkanethiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
groups that could sequester heavy metals
upright=1.2, Crystals of lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead
Heavy metals is a controversial and ambiguous term for metallic elements with relatively h ...
. However, there were some major limitations like, inhomogeneity of the pores compared to PMSs, and limited organic content (around 25% with respect to the silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
wall sites).
In 1999, reports described mesoporous organosilicas with organic groups located within the pore channel walls as "bridges" between Si centers. Since these materials had both organic and inorganic groups as integral part of the porous framework, they were considered as composites of organic and inorganic material and designated as periodic mesoporous organosilicas (PMOs). This family of porous materials had high degree of order and uniformity of pores compared to those with terminal organic groups.

Structure of PMOs
The framework of PMOs consists of inorganic components (polysilsesquioxanes) uniformly bridged by organic linkers. Most of the bridged polysilsesquioxane can be generically represented by the formula O1.5Si-R-SiO1.5. where R represents the organic bridging group. Each individual organic group is covalently bonded to two or more silicon atoms in the framework. The pores in the material are periodically ordered with diameter in the range 2 -30 nm. Depending on the synthetic conditions used to make mesoporous organosilicas, the mesoscale structure can either be amorphous or crystalline. Most of the mesoporous organosilicas that have been synthesized are amorphous. Although,x-ray diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. ...
of these materials indicate periodicity in the structure, sharp peaks in the medium scattering angle representative of crystalline materials are usually absent, except for (00l) reflections. However, few crystalline mesoporous organosilica have been reported,.

Synthesis
The primary methods used to make mesoporous organosilicas are evaporation-induced self-assembly, surfactant-mediated synthesis, post-synthetic grafting, and co-condensation. Organosilicas with amorphous structures are typically made by functionalizing organic groups rather than directly integrating the functional groups in the framework, which produces a periodic structure. Furthermore, basic hydrolytic conditions typically produce a periodic structure because of hydrophobic and hydrophilic interactions between hydrolyzed precursors that then self-assemble. Evaporation-inducedself-assembly
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the ...
usually causes random alignment of the material pores. This method of synthesis uses the difference in vapor pressure of solvents to vary the rate of evaporation and therefore the assembly of the organosilica framework.
Surfactant-mediated synthesis has been widely used for the production of mesoporous materials in general, and PMOs specifically,. It involves the addition of a surfactant
Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word ''surfactant'' is a Blend word, blend of "surface-active agent",
coined in ...
or copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are som ...
to a specific molecular precursor. The surfactant directs the structure of the material by interacting with the precursor in such a way that is dependent on the properties of the precursor. After the bulk structure is assembled, the surfactant is removed, leaving pores, or channels, embedded in the material framework. The surfactant template can be removed by solvent extraction or ion-exchange
Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of ch ...
mechanisms. An aging process is usually performed at high temperature before removal of the surfactant. During surfactant-mediated synthesis, hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
and polycondensation
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Natural proteins as well as s ...
, or co-condensation, are used to fuse precursor molecules in a framework. Acidic or basic conditions are used for the hydrolysis depending on the precursor being introduced.
The other two synthesis methods used for these materials are post-synthetic grafting and co-condensation. In the case of post-synthetic grafting, organic functional groups, typically organosilanes or alkoxyorganosilanes, are reacted with the assembled silicon mesostructure with or without the surfactant template present. If the template is still present, the grafting process will involve simultaneously removing the template and attaching the functional group. However, the pores of the material can be blocked during this process so a one-pot synthesis using the necessary components is more advantageous. This one-pot synthesis is known as co-condensation, in which the desired organosilyl functional groups are combined with the surfactant or other structure-directing agent. In this method, the material becomes structured and functionalized. Co-condensation gives rise to periodicity with the mesostructure, and it accommodates larger organic groups as well as larger pore sizes because of the one-step assembly process. Most PMOs have been made using the co-condensation method. The most recent method developed builds on co-condensation by combining multiple reactive organic precursors to form a new functional group, which is still combined with the framework molecule and copolymer.
Mesoporous organosilicate materials have been made using bridged organic precursors, in which an organic fragment is positioned between silicon-containing fragments. Single precursor syntheses are typically done with bridged organosilane groups. When only one bridged organic precursor is used, there is a homogeneous distribution of the molecule in the framework. This phenomenon is referred to as molecular-scale periodicity. Chiral precursors can also be introduced into the material framework, and using acidic conditions in the hydrolysis and condensation process proves better for chiral precursors because no racemization
In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred to as a racemic mixture (i.e. cont ...
occurs. Co-condensation of multiple organosilane precursors can create multi-functional organosilica materials. Tetraethoxysilane (TEOS) is a common silicon precursor used in co-condensation reactions.
Applications
Highly porous compounds are potentialcatalysts
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
, adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
, and separation. These have been the roles of zeolites, but their small pore size limits them to work with small molecules. The larger pore size (2-50 nm) of mesoporous materials gives them wider application – larger molecules can be admitted, and guest molecules can migrate faster.
Catalysis
To effect catalytic transformations using mesoporous organosilicas, it is necessary to functionalize them. The two major methods are to add a group orheteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
, such as a metal center, to the organic framework, and to anchor an organic or organometallic group to the pore surface.
 Anchoring a homogeneous catalyst onto a mesoporous organosilicas framework has two primary disadvantages: the bulky group in the pore can block travel of guest molecules through it, and preparation of candidate molecules for anchoring to the framework is difficult. However, anchoring can create heterogeneous catalysts for a wide variety of chemical transformations:
Anchoring a homogeneous catalyst onto a mesoporous organosilicas framework has two primary disadvantages: the bulky group in the pore can block travel of guest molecules through it, and preparation of candidate molecules for anchoring to the framework is difficult. However, anchoring can create heterogeneous catalysts for a wide variety of chemical transformations: acid catalysis
In acid catalysis and base catalysis, a chemical reaction is catalyzed by an acid or a base. By Brønsted–Lowry acid–base theory, the acid is the proton (hydrogen ion, H+) donor and the base is the proton acceptor. Typical reactions catalyz ...
, base catalysis, coupling
A coupling is a device used to connect two shafts together at their ends for the purpose of transmitting power. The primary purpose of couplings is to join two pieces of rotating equipment while permitting some degree of misalignment or end mo ...
and condensation reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a ...
catalysis, and even asymmetric catalysis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of Chirality (chemistry), chirality are formed ...
.
Anchored functional groups often have higher catalytic activity than does the bulk material, as one study showed for Nafion
Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer-copolymer synthesized in 1962 by Dr. Donald J. Connolly at the DuPont Experimental Station in Wilmington Delaware (U.S. Patent 3,282,875). Additional work on the polym ...
, or even than groups incorporated into the organosilica framework, as with sulfonic acid.
Other potential uses
 Mesoporous organosilicas can be functionalized give adsorbants, for removal specific contaminants from air and water. Candidate adsorbants include toxic heavy metals, radioactive material, and various organic pollutants have been synthesized.
Mesoporous organosilicas have been functionalized with fluorescent probes. The advantage of this material as a sensor is its high surface area combined with the high specificity achievable by careful functionalization. Mesoporous organosilicas have been used to sense a wide variety of analytes: metals, industrial pollutants, small organic molecules, and large biological molecules.
Mesoporous organosilicas have been tested as potential materials for separation using
Mesoporous organosilicas can be functionalized give adsorbants, for removal specific contaminants from air and water. Candidate adsorbants include toxic heavy metals, radioactive material, and various organic pollutants have been synthesized.
Mesoporous organosilicas have been functionalized with fluorescent probes. The advantage of this material as a sensor is its high surface area combined with the high specificity achievable by careful functionalization. Mesoporous organosilicas have been used to sense a wide variety of analytes: metals, industrial pollutants, small organic molecules, and large biological molecules.
Mesoporous organosilicas have been tested as potential materials for separation using HPLC
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can origina ...
. Froba ''et al.'' have shown that by using benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
PMO microspheres
Microparticles are particles between 0.1 and 100 μm in size. Commercially available microparticles are available in a wide variety of materials, including ceramics, glass, polymers, and metals. Microparticles encountered in daily life includ ...
as stationary phases better separation can be achieved in the HPLC system. The theory was that the π-π interaction between the aromatic analytes and the phenylene
In organic chemistry, the phenylene group () is based on a di-substituted benzene ring ( arylene). For example, poly(''p''-phenylene) is a polymer built up from ''para''-phenylene repeating units.p. C-9, Section 11.6, Handbook of Chemistry and ...
bridge of the PMO framework leads to stronger retention and hence better separation.
Controlled drug release is another aspect in which PMOs have been shown promise. The hydrophobic nature of the PMO walls allow for better control in drug release. In this respect, it is not just the mesoporosity of the PMOs make them advantageous, the tunability of the organic groups also play an important role.
Future directions
It has been proposed that the periodicity of PMOs may produceanisotropic
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit ver ...
mechanical, electrical and optical responses, in the same manner that periodicity magnifies anisotropy in the unit cell of conventional crystals. Also, studies that have shown that dendrimers
Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The ...
, polyhedral oligomeric silsesquioxanes, and carbon nanomaterials like C60 can be incorporated into the pore walls of PMOs offers new directions in the possible applications of these materials. It has been shown that PMOs are more suitable for the construction of organic donor–acceptor systems for photocatalysis
In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a photocatalyst, the excited state of which "repeatedly interacts with the reaction partners forming reaction intermediates and regenerates itself after each ...
than periodic mesoporous silica because organic donor or acceptor groups within the framework provide larger empty spaces for mass transfer in photocatalysis than in mesoporous silicas. Recent investigations on charge transfer systems based on PMOs are suggestive of possible applications of PMOs in areas as such as heterojunction solar cells
A solar cell, also known as a photovoltaic cell (PV cell), is an electronic device that converts the energy of light directly into electricity by means of the photovoltaic effect.
, photodetectors
Photodetectors, also called photosensors, are devices that detect light or other forms of electromagnetic radiation and convert it into an electrical signal. They are essential in a wide range of applications, from digital imaging and optical c ...
and light emitting diodes
A light-emitting diode (LED) is a semiconductor device that Light#Light sources, emits light when Electric current, current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of pho ...
. More exciting applications can emerge by combining these materials with biological molecules such as lipids and proteins.
PMOs with unconventional structures and properties have found high potential for future developments.
See also
*Mesoporous materials
A mesoporous material (or super nanoporous ) is a nanoporous material containing pores with diameters between 2 and 50 nm, according to IUPAC nomenclature. For comparison, IUPAC defines microporous material as a material having pores smalle ...
*Zeolites
Zeolites are a group of several Microporous material, microporous, crystalline aluminosilicate minerals commonly used as commercial adsorption, adsorbents and Catalysis, catalysts. They mainly consist of silicon, aluminium, oxygen, and have the ge ...
*Mesoporous silica
Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (50 ...
*Chirality
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable fro ...
* Metal-organic framework
*Asymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
References
{{Reflist, 30emExternal links
*http://sciencewatch.com/ana/st/mes-mat/ Porous media Silicon dioxide Organosilica