Meisenheimer Complex on:
[Wikipedia]
[Google]
[Amazon]
A Meisenheimer complex or Jackson–Meisenheimer complex in
 In 1902 Jakob Meisenheimer observed that by acidifying their reaction product, the starting material was recovered.
With three electron withdrawing groups, the negative charge in the complex is located at one of the nitro groups according to the quinoid model. When less electron poor arenes this charge is delocalized over the entire ring (structure to the right in ''scheme 1'').
In one study a Meisenheimer arene (4,6-dinitrobenzofuroxan) was allowed to react with a strongly electron-releasing arene (1,3,5-tris(N-pyrrolidinyl)benzene) forming a
In 1902 Jakob Meisenheimer observed that by acidifying their reaction product, the starting material was recovered.
With three electron withdrawing groups, the negative charge in the complex is located at one of the nitro groups according to the quinoid model. When less electron poor arenes this charge is delocalized over the entire ring (structure to the right in ''scheme 1'').
In one study a Meisenheimer arene (4,6-dinitrobenzofuroxan) was allowed to react with a strongly electron-releasing arene (1,3,5-tris(N-pyrrolidinyl)benzene) forming a 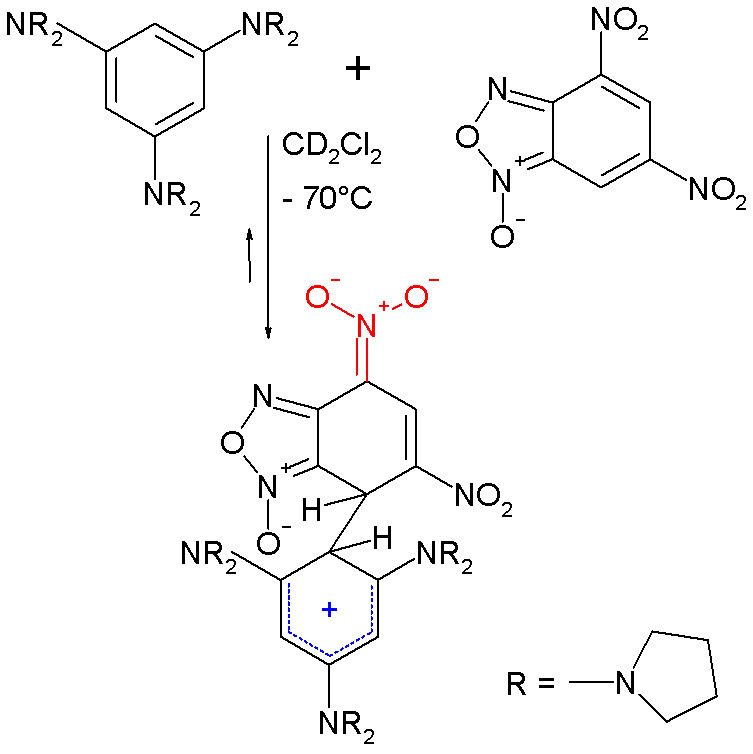 The structure of this complex was confirmed by
The structure of this complex was confirmed by

organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
is a 1:1 reaction adduct
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is ...
between an arene
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
carrying electron withdrawing groups and a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. These complexes are found as reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
s in nucleophilic aromatic substitution
A nucleophilic aromatic substitution (SNAr) is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic c ...
but stable and isolated Meisenheimer salts are also known.
Background
The early development of this type of complex takes place around the turn of the 19th century. In 1886 Janovski observed an intense violet color when he mixed ''meta''-dinitrobenzene with an alcoholic solution of alkali. In 1895Cornelis Adriaan Lobry van Troostenburg de Bruyn
Cornelis Adriaan Lobry van Troostenburg de Bruyn (1 January 1857 – 23 July 1904) was a chemist from the Netherlands.
Biography
De Bruyn was born on in Leeuwarden, where his father, Nicholaas Lobry van Troostenburg de Bruyn, was a physician in p ...
investigated a red substance formed in the reaction of trinitrobenzene Trinitrobenzenes are nitrobenzenes consisting of three nitro groups bonded to a central benzene ring.
There are three isomers of trinitrobenzene:
* 1,2,3-Trinitrobenzene
*
* 1,3,5-Trinitrobenzene
1,3,5-Trinitrobenzene is one of three isomers of ...
with potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utili ...
in methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
. In 1900 Jackson and Gazzolo reacted trinitroanisole
Trinitroanisole is a chemical compound that exists as pale yellow crystals with a melting point of 68 °C. It is highly toxic. It is an explosive with a detonation velocity of 7200 meters per second.Wasag-Chemie, Essen. "Explosivstoffe". 1961, p. ...
with sodium methoxide
Sodium methoxide is the simplest sodium alkoxide. With the formula , it is a white solid, which is formed by the deprotonation of methanol. It is a widely used reagent in industry and the laboratory. It is also a dangerously caustic base.
...
and proposed a quinoid structure for the reaction product.
:zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively and negatively charged functional groups.
:
(1,2- dipolar compounds, such as ylides, are sometimes excluded from ...
ic Meisenheimer–Wheland complex. The Wheland intermediate is the name typically given to the cationic reactive intermediate formed in electrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
, and can be considered an oppositely charged analog of the negatively charged Meisenheimer complex formed in nucleophilic aromatic substitution. Hence, the simultaneous occurrence of the Wheland and Meisenheimer intermediates in the single zwitterionic complex shown below lead to its description as a Meisenheimer–Wheland complex.
: The structure of this complex was confirmed by
The structure of this complex was confirmed by NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic f ...
.
Janovski reaction
The Janovski reaction is the reaction of 1,3-dinitrobenzene Dinitrobenzenes are nitrobenzenes composed of a benzene ring and two nitro group (-NO2) substituents. The three possible arrangements of the nitro groups afford three isomers, 1,2-dinitrobenzene, 1,3-dinitrobenzene, and 1,4-dinitrobenzene. Each iso ...
with an enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ...
izable ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
to the Meisenheimer adduct.
Zimmermann reaction
In the Zimmermann reaction the Janovski adduct is oxidized with excess base to a strongly colored enolate with subsequent reduction of the dinitro compound to the aromatic nitro amine. This reaction is the basis of the Zimmermann test used for the detection of ketosteroids.
Eponyms
The Jackson–Meisenheimer complex was named after the American organic chemist, Charles Loring Jackson (1847–1935) and the German organic chemist, Jakob Meisenheimer (1876–1934). The Janovski reaction was named for the Czech chemist, Jaroslav Janovski (1850–1907). The Zimmermann reaction was named after the German chemist, Wilhelm Zimmermann (1910–1982). Lastly, the Wheland intermediate was named after the American chemist, George Willard Wheland (1907–1976)References
{{Reflist, 40em Reactive intermediates Salts