Material Properties Of Diamond on:
[Wikipedia]
[Google]
[Amazon]
 Unlike hardness, which denotes only resistance to scratching, diamond's
Unlike hardness, which denotes only resistance to scratching, diamond's
Diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
is the allotrope of carbon in which the carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms are arranged in the specific type of cubic lattice called diamond cubic. It is a crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
that is transparent to opaque and which is generally isotropic (no or very weak birefringence
Birefringence, also called double refraction, is the optical property of a material having a refractive index that depends on the polarization and propagation direction of light. These optically anisotropic materials are described as birefrin ...
). Diamond is the hardest naturally occurring material known. Yet, due to important structural brittleness, bulk diamond's toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.tensile strength
Ultimate tensile strength (also called UTS, tensile strength, TS, ultimate strength or F_\text in notation) is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials, the ultimate ...
of bulk diamond is little known; however, compressive strength up to has been observed, and it could be as high as in the form of micro/nanometer-sized wires or needles (~ in diameter, micrometers long), with a corresponding maximum tensile elastic strain in excess of 9%. The anisotropy
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit ve ...
of diamond hardness is carefully considered during diamond cutting
Diamond cutting is the practice of shaping a Diamond (gemstone), diamond from a rough stone into a faceted gem. Cutting diamonds requires specialized knowledge, tools, equipment, and techniques because of its extreme difficulty.
The first guild ...
. Diamond has a high refractive index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ...
(2.417) and moderate dispersion (0.044) properties that give cut diamonds their brilliance. Scientists classify diamonds into four main types according to the nature of crystallographic defect
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in Crystal, crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the Crysta ...
s present. Trace impurities substitutionally replacing carbon atoms in a diamond's crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
, and in some cases structural defects, are responsible for the wide range of colors seen in diamond. Most diamonds are electrical insulators and extremely efficient thermal conductors. Unlike many other minerals, the specific gravity
Relative density, also called specific gravity, is a dimensionless quantity defined as the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for solids and liquids is nea ...
of diamond crystals (3.52) has rather small variation from diamond to diamond.
Hardness and crystal structure
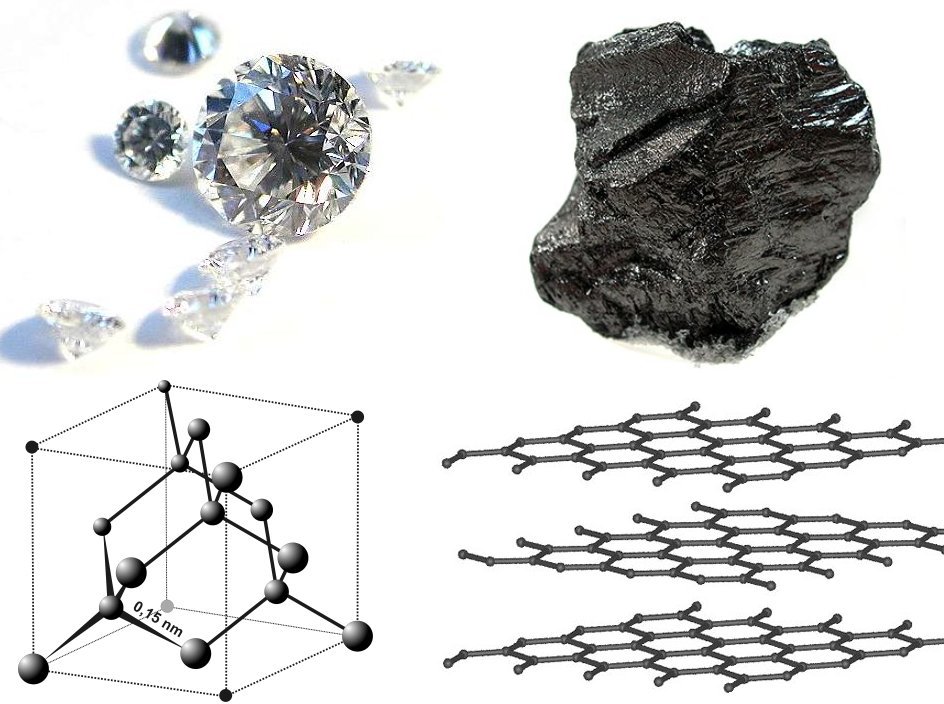
Known to the ancient Greeks as (, 'proper, unalterable, unbreakable') and sometimes called adamant, diamond is the hardest known naturally occurring material, and serves as the definition of 10 on the Mohs scale of mineral hardness. Diamond is extremely strong owing to its crystal structure, known as diamond cubic, in which each carbon atom has four neighbors covalently bonded to it. Bulk cubic boron nitride (c-BN) is nearly as hard as diamond. Diamond reacts with some materials, such as steel, and c-BN wears less when cutting or abrading such material. (Its zincblende structure is like the diamond cubic structure, but with alternating types of atoms.) A currently hypothetical material, beta carbon nitride (β-), may also be as hard or harder in one form. It has been shown that some diamond aggregates having nanometer grain size are harder and tougher than conventional large diamond crystals, thus they perform better as abrasive material. Owing to the use of those new ultra-hard materials for diamond testing, more accurate values are now known for diamond hardness. A surface perpendicular to the 11 crystallographic direction (that is the longest diagonal of a cube) of a pure (i.e., type IIa) diamond has a hardness value of when scratched with a nanodiamond tip, while the nanodiamond sample itself has a value of when tested with another nanodiamond tip. Because the test only works properly with a tip made of harder material than the sample being tested, the true value for nanodiamond is likely somewhat lower than . The precisetensile strength
Ultimate tensile strength (also called UTS, tensile strength, TS, ultimate strength or F_\text in notation) is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials, the ultimate ...
of diamond is unknown, though strength up to has been observed, and theoretically it could be as high as depending on the sample volume/size, the perfection of diamond lattice and on its orientation: Tensile strength is the highest for the 00crystal direction (normal to the cubic face), smaller for the 10and the smallest for the 11axis (along the longest cube diagonal). Diamond also has one of the smallest compressibilities of any material.
Cubic
Cubic may refer to:
Science and mathematics
* Cube (algebra), "cubic" measurement
* Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex
** Cubic crystal system, a crystal system w ...
diamonds have a perfect and easy octahedral cleavage, which means that they only have four planes—weak directions following the faces of the octahedron where there are fewer bonds—along which diamond can easily split upon blunt impact to leave a smooth surface. Similarly, diamond's hardness is markedly ''directional'': the hardest direction is the diagonal on the cube
A cube or regular hexahedron is a three-dimensional space, three-dimensional solid object in geometry, which is bounded by six congruent square (geometry), square faces, a type of polyhedron. It has twelve congruent edges and eight vertices. It i ...
face, 100 times harder than the softest direction, which is the dodecahedral plane. The octahedral plane is intermediate between the two extremes. The diamond cutting
Diamond cutting is the practice of shaping a Diamond (gemstone), diamond from a rough stone into a faceted gem. Cutting diamonds requires specialized knowledge, tools, equipment, and techniques because of its extreme difficulty.
The first guild ...
process relies heavily on this directional hardness, as without it a diamond would be nearly impossible to fashion. Cleavage also plays a helpful role, especially in large stones where the cutter wishes to remove flawed material or to produce more than one stone from the same piece of rough (e.g. Cullinan Diamond).
Diamonds crystallize in the diamond cubic crystal system ( space group Fdm) and consist of tetrahedrally, covalently bonded carbon atoms. A second form called lonsdaleite, with hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is d ...
symmetry, has also been found, but it is extremely rare and forms only in meteor
A meteor, known colloquially as a shooting star, is a glowing streak of a small body (usually meteoroid) going through Earth's atmosphere, after being heated to incandescence by collisions with air molecules in the upper atmosphere,
creating a ...
ites or in laboratory synthesis. The local environment of each atom is identical in the two structures. From theoretical considerations, lonsdaleite is expected to be harder than diamond, but the size and quality of the available stones are insufficient to test this hypothesis. In terms of crystal habit
In mineralogy, crystal habit is the characteristic external shape of an individual crystal or aggregate of crystals. The habit of a crystal is dependent on its crystallographic form and growth conditions, which generally creates irregularities d ...
, diamonds occur most often as euhedral (well-formed) or rounded octahedra and twinned, flattened octahedra with a triangular outline. Other forms include dodecahedra and (rarely) cubes. There is evidence that nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
impurities play an important role in the formation of well-shaped euhedral crystals. The largest diamonds found, such as the Cullinan Diamond, were shapeless. These diamonds are pure (i.e. type II) and therefore contain little if any nitrogen.
The faces of diamond octahedrons are highly lustrous owing to their hardness; triangular shaped growth defects (''trigons'') or ''etch pits'' are often present on the faces. A diamond's fracture
Fracture is the appearance of a crack or complete separation of an object or material into two or more pieces under the action of stress (mechanics), stress. The fracture of a solid usually occurs due to the development of certain displacemen ...
is irregular. Diamonds which are nearly round, due to the formation of multiple steps on octahedral faces, are commonly coated in a gum-like skin (''nyf''). The combination of stepped faces, growth defects, and nyf produces a "scaly" or corrugated appearance. Many diamonds are so distorted that few crystal faces are discernible. Some diamonds found in Brazil
Brazil, officially the Federative Republic of Brazil, is the largest country in South America. It is the world's List of countries and dependencies by area, fifth-largest country by area and the List of countries and dependencies by population ...
and the Democratic Republic of the Congo
The Democratic Republic of the Congo (DRC), also known as the DR Congo, Congo-Kinshasa, or simply the Congo (the last ambiguously also referring to the neighbouring Republic of the Congo), is a country in Central Africa. By land area, it is t ...
are polycrystalline and occur as opaque, darkly colored, spherical, radial masses of tiny crystals; these are known as ballas and are important to industry as they lack the cleavage planes of single-crystal diamond. Carbonado is a similar opaque microcrystalline form which occurs in shapeless masses. Like ballas diamond, carbonado lacks cleavage planes and its specific gravity varies widely from 2.9 to 3.5. Bort diamonds, found in Brazil, Venezuela
Venezuela, officially the Bolivarian Republic of Venezuela, is a country on the northern coast of South America, consisting of a continental landmass and many Federal Dependencies of Venezuela, islands and islets in the Caribbean Sea. It com ...
, and Guyana
Guyana, officially the Co-operative Republic of Guyana, is a country on the northern coast of South America, part of the historic British West Indies. entry "Guyana" Georgetown, Guyana, Georgetown is the capital of Guyana and is also the co ...
, are the most common type of industrial-grade diamond. They are also polycrystalline and often poorly crystallized; they are translucent and cleave easily.
Hydrophobia and lipophilia
Due to great hardness and strong molecular bonding, a cut diamond's facets and facet edges appear the flattest and sharpest. A curious side effect of a natural diamond's surface perfection is ''hydrophobia'' combined with ''lipophilia''. The former property means a drop of water placed on a diamond forms a coherent droplet, whereas in most other minerals the water would spread out to cover the surface. Additionally, diamond is unusually lipophilic, meaning grease and oil readily collect and spread on a diamond's surface, whereas in other minerals oil would form coherent drops. This property is exploited in the use ofgrease pencil
The grease pencil, a wax writing tool also known as a wax pencil, china marker, or chinagraph pencil (especially in the United Kingdom), is a writing implement made of hardened colored wax and is useful for marking on hard, glossy non-porous sur ...
s, which apply a line of grease to the surface of a suspect diamond simulant. Diamond surfaces are hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
when the surface carbon atoms terminate with a hydrogen atom and hydrophilic when the surface atoms terminate with an oxygen atom or hydroxyl radical. Treatment with gases or plasmas containing the appropriate gas, at temperatures of or higher, can change the surface property completely. Naturally occurring diamonds have a surface with less than a half monolayer coverage of oxygen, the balance being hydrogen and the behavior is moderately hydrophobic. This allows for separation from other minerals at the mine using the so-called "grease-belt".
Toughness
 Unlike hardness, which denotes only resistance to scratching, diamond's
Unlike hardness, which denotes only resistance to scratching, diamond's toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.brilliant cut of gemstones is designed specifically to reduce the likelihood of breakage or splintering.
Solid foreign crystals are commonly present in diamond. They are mostly minerals, such as
 Diamonds occur in various colors: black, brown, yellow, gray, white, blue, orange, purple to pink, and red. Colored diamonds contain
Diamonds occur in various colors: black, brown, yellow, gray, white, blue, orange, purple to pink, and red. Colored diamonds contain
 The luster of a diamond is described as " adamantine", which simply means diamond-like. Reflections on a properly cut diamond's facets are undistorted, due to their flatness. The
The luster of a diamond is described as " adamantine", which simply means diamond-like. Reflections on a properly cut diamond's facets are undistorted, due to their flatness. The
 If heated over in air, diamond, being a form of carbon, oxidizes and its surface blackens, but the surface can be restored by re-polishing. In absence of oxygen, e.g. in a flow of high-purity
If heated over in air, diamond, being a form of carbon, oxidizes and its surface blackens, but the surface can be restored by re-polishing. In absence of oxygen, e.g. in a flow of high-purity
Properties of diamondProperties of diamond
(S. Sque, PhD thesis, 2005, University of Exeter, UK) {{DEFAULTSORT:Diamond (material properties) Material properties of diamond Allotropes of carbon Native element minerals Superhard materials
olivine
The mineral olivine () is a magnesium iron Silicate minerals, silicate with the chemical formula . It is a type of Nesosilicates, nesosilicate or orthosilicate. The primary component of the Earth's upper mantle (Earth), upper mantle, it is a com ...
, garnet
Garnets () are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.
Garnet minerals, while sharing similar physical and crystallographic properties, exhibit a wide range of chemical compositions, de ...
s, ruby
Ruby is a pinkish-red-to-blood-red-colored gemstone, a variety of the mineral corundum ( aluminium oxide). Ruby is one of the most popular traditional jewelry gems and is very durable. Other varieties of gem-quality corundum are called sapph ...
, and many others. These and other inclusions, such as internal fractures or "feathers", can compromise the structural integrity of a diamond. Cut diamonds that have been enhanced to improve their clarity via glass infilling of fractures or cavities are especially fragile, as the glass will not stand up to ultrasonic cleaning or the rigors of the jeweler's torch. Fracture-filled diamonds may shatter if treated improperly.
Pressure resistance
Used in so-called diamond anvil experiments to create high-pressure environments, diamonds withstand crushing pressures in excess of 600 gigapascals (6 million atmospheres).Optical properties
Color and its causes
crystallographic defect
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in Crystal, crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the Crysta ...
s, including substitutional impurities and structural defects, that cause the coloration. Theoretically, pure diamonds would be transparent and colorless. Diamonds are scientifically classed into two main ''types'' and several subtypes, according to the nature of defects present and how they affect light absorption:
Type I diamond has nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
(N) atoms as the main impurity, at a concentration of up to 1%. If the N atoms are in pairs or larger aggregates, they do not affect the diamond's color; these are Type Ia. About 98% of gem diamonds are type Ia: these diamonds belong to the ''Cape series'', named after the diamond-rich region formerly known as Cape Province in South Africa
South Africa, officially the Republic of South Africa (RSA), is the Southern Africa, southernmost country in Africa. Its Provinces of South Africa, nine provinces are bounded to the south by of coastline that stretches along the Atlantic O ...
, whose deposits are largely Type Ia. If the nitrogen atoms are dispersed throughout the crystal in isolated sites (not paired or grouped), they give the stone an intense yellow or occasionally brown tint (type Ib); the rare canary diamonds belong to this type, which represents only ~0.1% of known natural diamonds. Synthetic diamond containing nitrogen is usually of type Ib. Type Ia and Ib diamonds absorb in both the infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
and ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
region of the electromagnetic spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high ...
, from . They also have a characteristic fluorescence and visible absorption spectrum.
Type II diamonds have very few if any nitrogen impurities. Pure (type IIa) diamond can be colored pink, red, or, brown owing to structural anomalies arising through ''plastic deformation'' during crystal growth; these diamonds are rare (1.8% of gem diamonds), but constitute a large percentage of Australian diamonds. Type IIb diamonds, which account for ~0.1% of gem diamonds, are usually a steely blue or gray due to boron atoms scattered within the crystal matrix. These diamonds are also semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
s, unlike other diamond types (see Electrical properties). Most blue-gray diamonds coming from the Argyle mine of Australia are not of type IIb, but of Ia type. Those diamonds contain large concentrations of defects and impurities (especially hydrogen and nitrogen) and the origin of their color is yet uncertain. Type II diamonds weakly absorb in a different region of the infrared (the absorption is due to the diamond lattice rather than impurities), and transmit in the ultraviolet below 225 nm, unlike type I diamonds. They also have differing fluorescence characteristics, but no discernible visible absorption spectrum.
Certain diamond enhancement techniques are commonly used to artificially produce an array of colors, including blue, green, yellow, red, and black. Color enhancement techniques usually involve irradiation, including proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
bombardment via cyclotron
A cyclotron is a type of particle accelerator invented by Ernest Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Januar ...
s; neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
bombardment in the piles of nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s; and electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
bombardment by Van de Graaff generator
A Van de Graaff generator is an electrostatic generator which uses a moving belt to accumulate electric charge on a hollow metal globe on the top of an insulated column, creating very high electric potentials. It produces very high voltage direct ...
s. These high-energy particles physically alter the diamond's crystal lattice
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystal, crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that ...
, knocking carbon atoms out of place and producing color centers. The depth of color penetration depends on the technique and its duration, and in some cases the diamond may be left radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
to some degree.
Some irradiated diamonds are completely natural; one famous example is the Dresden Green Diamond. In these natural stones the color is imparted by "radiation burns" (natural irradiation by alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s originating from uranium ore) in the form of small patches, usually only micrometers deep. Additionally, Type IIa diamonds can have their structural deformations "repaired" via a high-pressure high-temperature (HPHT) process, removing much or all of the diamond's color.
Luster
 The luster of a diamond is described as " adamantine", which simply means diamond-like. Reflections on a properly cut diamond's facets are undistorted, due to their flatness. The
The luster of a diamond is described as " adamantine", which simply means diamond-like. Reflections on a properly cut diamond's facets are undistorted, due to their flatness. The refractive index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ...
of diamond (as measured via sodium light, ) is 2.417. Because it is cubic in structure, diamond is also isotropic
In physics and geometry, isotropy () is uniformity in all orientations. Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence '' anisotropy''. ''Anisotropy'' is also ...
. Its high dispersion of 0.044 (variation of refractive index across the visible spectrum) manifests in the perceptible ''fire'' of cut diamonds. This fire—flashes of prismatic colors seen in transparent stones—is perhaps diamond's most important optical property from a jewelry perspective. The prominence or amount of fire seen in a stone is heavily influenced by the choice of diamond cut
A diamond cut is a style or design guide used when shaping a diamond for polishing such as the Brilliant (diamond cut), brilliant cut. Cut refers to shape (Pear cut, pear, oval), and also the symmetry, proportioning and polish of a diamond. The ...
and its associated proportions (particularly crown height), although the body color of fancy (i.e., unusual) diamonds may hide their fire to some degree.
More than 20 other minerals have higher dispersion (that is difference in refractive index for blue and red light) than diamond, such as titanite 0.051, andradite 0.057, cassiterite 0.071, strontium titanate 0.109, sphalerite
Sphalerite is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimentary exhalative, Carbonate-hoste ...
0.156, synthetic rutile
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at vis ...
0.330, cinnabar
Cinnabar (; ), or cinnabarite (), also known as ''mercurblende'' is the bright scarlet to brick-red form of Mercury sulfide, mercury(II) sulfide (HgS). It is the most common source ore for refining mercury (element), elemental mercury and is t ...
0.4, etc. (see Dispersion (optics)). However, the combination of dispersion with extreme hardness, wear and chemical resistivity, as well as clever marketing, determines the exceptional value of diamond as a gemstone.
Fluorescence
Diamonds exhibitfluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
, that is, they emit light of various colors and intensities under long-wave ultraviolet light (365 nm): Cape series stones (type Ia) usually fluoresce blue, and these stones may also phosphoresce yellow, a unique property among gemstones. Other possible long-wave fluorescence colors are green (usually in brown stones), yellow, mauve, or red (in type IIb diamonds). In natural diamonds, there is typically little if any response to short-wave ultraviolet, but the reverse is true of synthetic diamonds. Some natural type IIb diamonds phosphoresce blue after exposure to short-wave ultraviolet. In natural diamonds, fluorescence under X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
s is generally bluish-white, yellowish or greenish. Some diamonds, particularly Canadian diamonds, show no fluorescence.
The origin of the luminescence colors is often unclear and not unique. Blue emission from type IIa and IIb diamonds is reliably identified with dislocations by directly correlating the emission with dislocations in an electron microscope
An electron microscope is a microscope that uses a beam of electrons as a source of illumination. It uses electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing it ...
. However, blue emission in type Ia diamond could be either due to dislocations or the N3 defects (three nitrogen atoms bordering a vacancy). Green emission in natural diamond is usually due to the H3 center (two substitutional nitrogen atoms separated by a vacancy), whereas in synthetic diamond it usually originates from nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
used as a catalyst (see figure). Orange or red emission could be due to various reasons, one being the nitrogen-vacancy center which is present in sufficient quantities in all types of diamond, even type IIb.
Optical absorption
Cape series (Ia) diamonds have a visible absorption spectrum (as seen through a direct-vision spectroscope) consisting of a fine line in the violet at ; however, this line is often invisible until the diamond has been cooled to very low temperatures. Associated with this are weaker lines at , , , , and . All those lines are labeled as N3 and N2 optical centers and associated with a defect consisting of three nitrogen atoms bordering a vacancy. Other stones show additional bands: brown, green, or yellow diamonds show a band in the green at (H3 center, see above), sometimes accompanied by two additional weak bands at and (H4 center, a large complex presumably involving 4 substitutional nitrogen atoms and 2 lattice vacancies). Type IIb diamonds may absorb in the far red due to the substitutional boron, but otherwise show no observable visible absorption spectrum. Gemological laboratories make use of spectrophotometer machines that can distinguish natural, artificial, and color- enhanced diamonds. The spectrophotometers analyze theinfrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
, visible, and ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
absorption and luminescence spectra of diamonds cooled with liquid nitrogen
Liquid nitrogen (LN2) is nitrogen in a liquid state at cryogenics, low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose vis ...
to detect tell-tale absorption lines that are not normally discernible.
Electrical properties
Diamond is a good electrical insulator, having a resistivity of to ( – ), and is famous for its widebandgap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to the ...
of 5.47 eV. High carrier mobilities and high electric breakdown field at room temperature are also important characteristics of diamond. Those characteristics allow single crystalline diamond to be one of the promising materials for semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
s. A wide bandgap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to the ...
is advantageous in semiconductors because it allows them to maintain high resistivity even at high temperature, important for high power applications. Semiconductors whose carrier mobilities are high such as diamond are easier to utilize in industry because they do not need high input voltage. High breakdown voltage avoids a huge current suddenly occurring at typical input voltages.
Most natural blue diamonds are an exception and are semiconductors due to substitutional boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
impurities replacing carbon atoms. Natural blue or blue-gray diamonds, common for the Argyle diamond mine in Australia, are rich in hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
; these diamonds are not semiconductors and it is unclear whether hydrogen is actually responsible for their blue-gray color. Natural blue diamonds containing boron and synthetic diamonds doped with boron are p-type semiconductors. N-type diamond films are reproducibly synthesized by phosphorus doping during chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (electro ...
. Diode p-n junctions and UV light emitting diodes ( LEDs, at ) have been produced by sequential deposition of p-type (boron-doped) and n-type (phosphorus-doped) layers.
Diamond's electronic properties can be also modulated by strain engineering.
Diamond transistor
A transistor is a semiconductor device used to Electronic amplifier, amplify or electronic switch, switch electrical signals and electric power, power. It is one of the basic building blocks of modern electronics. It is composed of semicondu ...
s have been produced (for research purposes). In January 2024, a Japanese research team fabricated a MOSFET
upright=1.3, Two power MOSFETs in amperes">A in the ''on'' state, dissipating up to about 100 watt">W and controlling a load of over 2000 W. A matchstick is pictured for scale.
In electronics, the metal–oxide–semiconductor field- ...
using phosphorus-doped n-type diamond, which would have superior characteristics to silicon-based technology in high-temperature, high-frequency or high-electron mobility applications. FETs with SiN dielectric layers, and SC-FETs have been made.
In April 2004, research published in the journal Nature
Nature is an inherent character or constitution, particularly of the Ecosphere (planetary), ecosphere or the universe as a whole. In this general sense nature refers to the Scientific law, laws, elements and phenomenon, phenomena of the physic ...
reported that below , synthetic boron-doped diamond is a bulk superconductor. Superconductivity was later observed in heavily boron-doped films grown by various chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (electro ...
techniques, and the highest reported transition temperature (by 2009) is . (See also Covalent superconductor#Diamond)
Uncommon magnetic properties (spin glass state) were observed in diamond nanocrystals intercalated with potassium. Unlike paramagnetic host material, magnetic susceptibility measurements of intercalated nanodiamond revealed distinct ferromagnetic behavior at . This is essentially different from results of potassium intercalation in graphite or C60 fullerene, and shows that sp3 bonding promotes magnetic ordering in carbon. The measurements presented first experimental evidence of intercalation-induced spin-glass state in a nanocrystalline diamond system.
Thermal conductivity
Unlike most electrical insulators, diamond is a good conductor of heat because of covalent bonding. Thermal conductivity of natural diamond was measured to be about 2,200 W/(m·K), which is five times more thansilver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
, the most thermally conductive metal. Monocrystalline synthetic diamond enriched to 99.9% the isotope 12C had the highest thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
of any known solid at room temperature: 3,320 W/(m·K), though reports exist of superior thermal conductivity in both carbon nanotubes and graphene. Because diamond has such high thermal conductance it is already used in semiconductor manufacture to prevent silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
and other semiconducting materials from overheating. At lower temperatures conductivity becomes even better, and reaches 41,000 W/(m·K) at (12C-enriched diamond).
Diamond's high thermal conductivity is used by jewelers and gemologists who may employ an electronic ''thermal probe'' to distinguish diamonds from their imitations. These probes consist of a pair of battery-powered thermistors mounted in a fine copper tip. One thermistor functions as a heating device while the other measures the temperature of the copper tip: if the stone being tested is a diamond, it will conduct the tip's thermal energy rapidly enough to produce a measurable temperature drop. This test takes about 2–3 seconds. However, older probes will be fooled by moissanite, a crystalline mineral form of silicon carbide
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A wide bandgap semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder a ...
introduced in 1998 as an alternative to diamonds, which has a similar thermal conductivity.
Technologically, the high thermal conductivity of diamond is used for the efficient heat removal in high-end power electronics. Diamond is especially appealing in situations where electrical conductivity of the heat sinking material cannot be tolerated e.g. for the thermal management of high-power radio-frequency
Radio frequency (RF) is the oscillation rate of an alternating electric current or voltage or of a magnetic, electric or electromagnetic field or mechanical system in the frequency range from around to around . This is roughly between the ...
() microcoils that are used to produce strong and local RF fields.
Thermal stability
 If heated over in air, diamond, being a form of carbon, oxidizes and its surface blackens, but the surface can be restored by re-polishing. In absence of oxygen, e.g. in a flow of high-purity
If heated over in air, diamond, being a form of carbon, oxidizes and its surface blackens, but the surface can be restored by re-polishing. In absence of oxygen, e.g. in a flow of high-purity argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
gas, diamond can be heated up to about . At high pressure (~) diamond can be heated up to , and a report published in 2009 suggests that diamond can withstand temperatures of and above.
Diamonds are carbon crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s that form under high temperatures and extreme pressures such as deep within the Earth. At surface air pressure (one atmosphere), diamonds are not as stable as graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
, and so the decay of diamond is thermodynamically favorable (δ''H'' = ). However, owing to a very large kinetic energy
In physics, the kinetic energy of an object is the form of energy that it possesses due to its motion.
In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2.Resnick, Rober ...
barrier, diamonds are metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
; they will not decay into graphite under normal conditions.
See also
* Chemical vapor deposition of diamond * Crystallographic defects in diamond * Nitrogen-vacancy center *Synthetic diamond
A synthetic diamond or laboratory-grown diamond (LGD), also called a lab-grown, laboratory-created, man-made, artisan-created, artificial, or cultured diamond, is a diamond that is produced in a controlled technological process, in contrast to ...
References
Further reading
*Pagel-Theisen, Verena. (2001). ''Diamond grading ABC: The manual'' (9th ed.), pp. 84–85. Rubin & Son n.v.; Antwerp, Belgium. *Webster, Robert, and Jobbins, E. Allan (Ed.). (1998). ''Gemmologist's compendium'', p. 21, 25, 31. St Edmundsbury Press Ltd, Bury St Edwards.External links
Properties of diamond
(S. Sque, PhD thesis, 2005, University of Exeter, UK) {{DEFAULTSORT:Diamond (material properties) Material properties of diamond Allotropes of carbon Native element minerals Superhard materials