material balance on:
[Wikipedia]
[Google]
[Amazon]
In
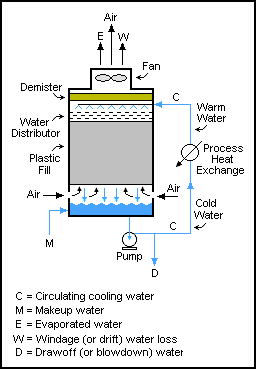 Mass balances can be performed across systems which have cyclic flows. In these systems output streams are fed back into the input of a unit, often for further reprocessing.
Such systems are common in grinding circuits, where grain is crushed then sieved to only allow fine particles out of the circuit and the larger particles are returned to the roller mill (grinder). However, recycle flows are by no means restricted to
Mass balances can be performed across systems which have cyclic flows. In these systems output streams are fed back into the input of a unit, often for further reprocessing.
Such systems are common in grinding circuits, where grain is crushed then sieved to only allow fine particles out of the circuit and the larger particles are returned to the roller mill (grinder). However, recycle flows are by no means restricted to
Material Balance Calculations
Material Balance Fundamentals
The Material Balance for Chemical Reactors
Material and energy balance
* {{cite book , last = Morris , first = Arthur E. , last2 = Geiger , first2 = Gordon , last3 = Fine , first3 = H. Alan , title = Handbook on Material and Energy Balance Calculations in Material Processing , url = http://www.wiley.com/WileyCDA/WileyTitle/productCd-1118065654.html , edition = 3rd , date = 2011 , isbn = 978-1-118-06565-5 , publisher = Wiley Mass Chemical process engineering Transport phenomena
physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge whi ...
, a mass balance, also called a material balance, is an application of conservation of mass
In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter the mass of the system must remain constant over time.
The law implies that mass can neith ...
to the analysis of physical systems
A physical system is a collection of physical objects under study. The collection differs from a set: all the objects must coexist and have some physical relationship.
In other words, it is a portion of the physical universe chosen for analysi ...
. By accounting for material entering and leaving a system, mass flows can be identified which might have been unknown, or difficult to measure without this technique. The exact conservation law
In physics, a conservation law states that a particular measurable property of an isolated physical system does not change as the system evolves over time. Exact conservation laws include conservation of mass-energy, conservation of linear momen ...
used in the analysis of the system depends on the context of the problem, but all revolve around mass conservation, i.e., that matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic pa ...
cannot disappear or be created spontaneously.
Therefore, mass balances are used widely in engineering
Engineering is the practice of using natural science, mathematics, and the engineering design process to Problem solving#Engineering, solve problems within technology, increase efficiency and productivity, and improve Systems engineering, s ...
and environmental analyses. For example, mass balance theory is used to design chemical reactor
A chemical reactor is an enclosed volume in which a chemical reaction takes place. In chemical engineering, it is generally understood to be a process vessel used to carry out a chemical reaction, which is one of the classic unit operations in che ...
s, to analyse alternative processes to produce chemicals, as well as to model pollution
Pollution is the introduction of contaminants into the natural environment that cause harm. Pollution can take the form of any substance (solid, liquid, or gas) or energy (such as radioactivity, heat, sound, or light). Pollutants, the component ...
dispersion and other processes of physical systems. Mass balances form the foundation of process engineering
Process engineering is a field of study focused on the development and optimization of industrial processes. It consists of the understanding and application of the fundamental principles and laws of nature to allow humans to transform raw mate ...
design. Closely related and complementary analysis techniques include the population balance, energy balance and the somewhat more complex entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
balance. These techniques are required for thorough design and analysis of systems such as the refrigeration cycle.
In environmental monitoring
Environmental monitoring is the processes and activities that are done to characterize and describe the state of the environment. It is used in the preparation of environmental impact assessments, and in many circumstances in which human activit ...
, the term budget calculations is used to describe mass balance equations where they are used to evaluate the monitoring data (comparing input and output, etc.). In biology
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, History of life, origin, evolution, and ...
, the dynamic energy budget
The dynamic energy budget (DEB) theory is a formal metabolic theory which provides a single quantitative framework to dynamically describe the aspects of metabolism (energy and mass budgets) of all living organisms at the individual level, based o ...
theory for metabolic organisation makes explicit use of mass and energy balance.
Introduction
The general form quoted for a mass balance is ''The mass that enters a system must, by conservation of mass, either leave the system or accumulate within the system''. Mathematically the mass balance for a system without a chemical reaction is as follows: Strictly speaking the above equation holds also for systems withchemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
s if the terms in the balance equation are taken to refer to total mass, i.e. the sum of all the chemical species of the system. In the absence of a chemical reaction the amount of any chemical species flowing in and out will be the same; this gives rise to an equation for each species present in the system. However, if this is not the case then the mass balance equation must be amended to allow for the generation or depletion (consumption) of each chemical species. Some use one term in this equation to account for chemical reactions, which will be negative for depletion and positive for generation. However, the conventional form of this equation is written to account for both a positive generation term (i.e. product of reaction) and a negative consumption term (the reactants used to produce the products). Although overall one term will account for the total balance on the system, if this balance equation is to be applied to an individual species and then the entire process, both terms are necessary. This modified equation can be used not only for reactive systems, but for population balances such as arise in particle mechanics problems. The equation is given below; note that it simplifies to the earlier equation in the case that the generation term is zero.
*In the absence of a nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
the number of atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
flowing in and out must remain the same, even in the presence of a chemical reaction.
*For a balance to be formed, the boundaries of the system must be clearly defined.
*Mass balances can be taken over physical systems at multiple scales.
*Mass balances can be simplified with the assumption of steady state
In systems theory, a system or a process is in a steady state if the variables (called state variables) which define the behavior of the system or the process are unchanging in time. In continuous time, this means that for those properties ''p' ...
, in which the accumulation term is zero.
Illustrative example
A simple example can illustrate the concept. Consider the situation in which aslurry
A slurry is a mixture of denser solids suspended in liquid, usually water. The most common use of slurry is as a means of transporting solids or separating minerals, the liquid being a carrier that is pumped on a device such as a centrifugal pu ...
is flowing into a settling tank
Settling is the process by which particulates move towards the bottom of a liquid and form a sediment. Particles that experience a force, either due to gravity or due to centrifugal motion will tend to move in a uniform manner in the direction ...
to remove the solids in the tank. Solids are collected at the bottom by means of a conveyor belt
A conveyor belt is the carrying medium of a belt conveyor system (often shortened to a belt conveyor). A belt conveyor system consists of two or more pulleys (sometimes referred to as drums), with a closed loop of carrying medium—the conveyor b ...
partially submerged in the tank, and water exits via an overflow outlet.
In this example, there are two substances: solids and water. The water overflow outlet carries an increased concentration of water relative to solids, as compared to the slurry inlet, and the exit of the conveyor belt carries an increased concentration of solids relative to water.
Assumptions
*Steady state
*Non-reactive system
Analysis
Suppose that the slurry inlet composition (by mass) is 50% solid and 50% water, with a mass flow of . The tank is assumed to be operating at steady state, and as such accumulation is zero, so input and output must be equal for both the solids and water. If we know that the removal efficiency for the slurry tank is 60%, then the water outlet will contain of solids (40% times times 50% solids). If we measure the flow rate of the combined solids and water, and the water outlet is shown to be , then the amount of water exiting via the conveyor belt must be . This allows us to completely determine how the mass has been distributed in the system with only limited information and using the mass balance relations across the system boundaries. The mass balance for this system can be described in a tabular form:
Mass feedback (recycle)
 Mass balances can be performed across systems which have cyclic flows. In these systems output streams are fed back into the input of a unit, often for further reprocessing.
Such systems are common in grinding circuits, where grain is crushed then sieved to only allow fine particles out of the circuit and the larger particles are returned to the roller mill (grinder). However, recycle flows are by no means restricted to
Mass balances can be performed across systems which have cyclic flows. In these systems output streams are fed back into the input of a unit, often for further reprocessing.
Such systems are common in grinding circuits, where grain is crushed then sieved to only allow fine particles out of the circuit and the larger particles are returned to the roller mill (grinder). However, recycle flows are by no means restricted to solid mechanics
Solid mechanics (also known as mechanics of solids) is the branch of continuum mechanics that studies the behavior of solid materials, especially their motion and deformation (mechanics), deformation under the action of forces, temperature chang ...
operations; they are used in liquid and gas flows, as well. One such example is in cooling tower
A cooling tower is a device that rejects waste heat to the atmosphere through the cooling of a coolant stream, usually a water stream, to a lower temperature. Cooling towers may either use the evaporation of water to remove heat and cool the ...
s, where water is pumped through a tower many times, with only a small quantity of water drawn off at each pass (to prevent solids build up) until it has either evaporated or exited with the drawn off water. The mass balance for water is .
The use of the recycle aids in increasing overall conversion of input products, which is useful for low per-pass conversion processes (such as the Haber process
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the ammonia production, production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using finely di ...
).
Differential mass balances
A mass balance can also be taken differentially. The concept is the same as for a large mass balance, but it is performed in the context of a limiting system (for example, one can consider the limiting case in time or, more commonly, volume). A differential mass balance is used to generate differential equations that can provide an effective tool for modelling and understanding the target system. The differential mass balance is usually solved in two steps: first, a set of governing differential equations must be obtained, and then these equations must be solved, either analytically or, for less tractable problems, numerically. The following systems are good examples of the applications of the differential mass balance: # Ideal (stirred) batch reactor # Ideal tank reactor, also namedContinuous Stirred Tank Reactor
The continuous stirred-tank reactor (CSTR), also known as vat- or backmix reactor, mixed flow reactor (MFR), or a continuous-flow stirred-tank reactor (CFSTR), is a common model for a chemical reactor in chemical engineering and environmental eng ...
(CSTR)
# Ideal Plug Flow Reactor (PFR)
Ideal batch reactor
The ideal completely mixed batch reactor is a closed system. Isothermal conditions are assumed, and mixing prevents concentration gradients as reactant concentrations decrease and product concentrations increase over time. Many chemistry textbooks implicitly assume that the studied system can be described as a batch reactor when they write about reaction kinetics andchemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable chan ...
.
The mass balance for a substance A becomes
where
* denotes the rate at which substance A is produced;
* is the volume (which may be constant or not);
* the number of moles () of substance A.
In a fed-batch reactor some reactants/ingredients are added continuously or in pulses (compare making porridge by either first blending all ingredients and then letting it boil, which can be described as a batch reactor, or by first mixing only water and salt and making that boil before the other ingredients are added, which can be described as a fed-batch reactor). Mass balances for fed-batch reactors become a bit more complicated.
Reactive example
In the first example, we will show how to use a mass balance to derive a relationship between the percent excess air for thecombustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion ...
of a hydrocarbon-base fuel oil and the percent oxygen in the combustion product gas. First, normal dry air contains of oxygen per mole of air, so there is one mole of in of dry air. For stoichiometric
Stoichiometry () is the relationships between the masses of reactants and products before, during, and following chemical reactions.
Stoichiometry is based on the law of conservation of mass; the total mass of reactants must equal the total m ...
combustion, the relationships between the mass of air and the mass of each combustible element in a fuel oil are:
Considering the accuracy of typical analytical procedures, an equation for the mass of air per mass of fuel at stoichiometric combustion is:
where refer to the mass fraction of each element in the fuel oil, sulfur burning to , and refers to the air-fuel ratio in mass units.
For of fuel oil containing 86.1% C, 13.6% H, 0.2% O, and 0.1% S the stoichiometric mass of air is , so AFR = 14.56. The combustion product mass is then . At exact stoichiometry, should be absent. At 15 percent excess air, the AFR = 16.75, and the mass of the combustion product gas is , which contains of excess oxygen. The combustion gas thus contains 2.84 percent by mass. The relationships between percent excess air and % in the combustion gas are accurately expressed by quadratic equations, valid over the range 0–30 percent excess air:
In the second example, we will use the law of mass action
In chemistry, the law of mass action is the proposition that the rate of a chemical reaction is directly proportional to the product of the activities or concentrations of the reactants. It explains and predicts behaviors of solutions in dy ...
to derive the expression for a chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable chan ...
constant.
Assume we have a closed reactor in which the following liquid phase reversible reaction occurs:
The mass balance for substance A becomes
As we have a liquid phase reaction we can (usually) assume a constant volume and since we get
or
In many textbooks this is given as the definition of reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
without specifying the implicit assumption that we are talking about reaction rate in a closed system with only one reaction. This is an unfortunate mistake that has confused many students over the years.
According to the law of mass action
In chemistry, the law of mass action is the proposition that the rate of a chemical reaction is directly proportional to the product of the activities or concentrations of the reactants. It explains and predicts behaviors of solutions in dy ...
the forward reaction rate can be written as
and the backward reaction rate as
The rate at which substance A is produced is thus
and since, at equilibrium, the concentration of A is constant we get
or, rearranged
Ideal tank reactor/continuously stirred tank reactor
The continuously mixed tank reactor is an open system with an influent stream of reactants and an effluent stream of products. A lake can be regarded as a tank reactor, and lakes with long turnover times (e.g. with low flux-to-volume ratios) can for many purposes be regarded as continuously stirred (e.g. homogeneous in all respects). The mass balance then becomes where * is the volumetric flow ''into'' the system; * is the volumetric flow ''out'' of the system; * is the concentration of A in the ''inflow''; * is the concentration of A in the ''outflow''. In an open system we can never reach a chemical equilibrium. We can, however, reach asteady state
In systems theory, a system or a process is in a steady state if the variables (called state variables) which define the behavior of the system or the process are unchanging in time. In continuous time, this means that for those properties ''p' ...
where all state variables (temperature, concentrations, etc.) remain constant ().
Example
Consider a bathtub in which there is some bathing salt dissolved. We now fill in more water, keeping the bottom plug in. What happens? Since there is no reaction, and since there is no outflow . The mass balance becomes or Using a mass balance for total volume, however, it is evident that and that Thus we get Note that there is no reaction and hence noreaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
or rate law involved, and yet . We can thus draw the conclusion that reaction rate can not be defined in a general manner using . One must first write down a mass balance before a link between and the reaction rate can be found. Many textbooks, however, define reaction rate as
without mentioning that this definition implicitly assumes that the system is closed, has a constant volume and that there is only one reaction.
Ideal plug flow reactor (PFR)
The idealized plug flow reactor is an open system resembling a tube with no mixing in the direction of flow but perfect mixing perpendicular to the direction of flow, often used for systems like rivers and water pipes if the flow is turbulent. When a mass balance is made for a tube, one first considers aninfinitesimal
In mathematics, an infinitesimal number is a non-zero quantity that is closer to 0 than any non-zero real number is. The word ''infinitesimal'' comes from a 17th-century Modern Latin coinage ''infinitesimus'', which originally referred to the " ...
part of the tube and make a mass balance over that using the ideal tank reactor model. That mass balance is then integrated over the entire reactor volume to obtain:
In numeric solutions, e.g. when using computers, the ideal tube is often translated to a series of tank reactors, as it can be shown that a PFR is equivalent to an infinite number of stirred tanks in series, but the latter is often easier to analyze, especially at steady state.
More complex problems
In reality, reactors are often non-ideal, in which combinations of the reactor models above are used to describe the system. Not only chemical reaction rates, but alsomass transfer
Mass transfer is the net movement of mass from one location (usually meaning stream, phase, fraction, or component) to another. Mass transfer occurs in many processes, such as absorption, evaporation, drying, precipitation, membrane filtra ...
rates may be important in the mathematical description of a system, especially in heterogeneous
Homogeneity and heterogeneity are concepts relating to the uniformity of a substance, process or image. A homogeneous feature is uniform in composition or character (i.e., color, shape, size, weight, height, distribution, texture, language, i ...
systems.
As the chemical reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
depends on temperature it is often necessary to make both an energy balance (often a heat balance rather than a full-fledged energy balance) as well as mass balances to fully describe the system. A different reactor model might be needed for the energy balance: A system that is closed with respect to mass might be open with respect to energy e.g. since heat may enter the system through conduction
Conductor or conduction may refer to:
Biology and medicine
* Bone conduction, the conduction of sound to the inner ear
* Conduction aphasia, a language disorder
Mathematics
* Conductor (ring theory)
* Conductor of an abelian variety
* Condu ...
.
Commercial use
In industrial process plants, using the fact that the mass entering and leaving any portion of a process plant must balance, data validation and reconciliation algorithms may be employed to correct measured flows, provided that enough redundancy of flow measurements exist to permit statistical reconciliation and exclusion of detectably erroneous measurements. Since all real world measured values contain inherent error, the reconciled measurements provide a better basis than the measured values do for financial reporting, optimization, and regulatory reporting. Software packages exist to make this commercially feasible on a daily basis.See also
*Bioreactor
A bioreactor is any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical reaction, chemical process is carried out which involves organisms or biochemistry, biochem ...
* Chemical engineering
Chemical engineering is an engineering field which deals with the study of the operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials ...
* Continuity equation
A continuity equation or transport equation is an equation that describes the transport of some quantity. It is particularly simple and powerful when applied to a conserved quantity, but it can be generalized to apply to any extensive quantity ...
* Dilution (equation)
* Energy accounting Energy accounting is a system used to measure, analyze and report the energy consumption of different activities on a regular basis. This is done to improve energy efficiency, and to monitor the environment impact of energy consumption.
Energy man ...
* Glacier mass balance
* Mass flux
In physics and engineering, mass flux is the rate of mass flow per unit of area. Its SI units are kgs−1m−2. The common symbols are ''j'', ''J'', ''q'', ''Q'', ''φ'', or Φ (Greek lowercase or capital Phi), sometimes with subscript ''m'' to i ...
* Material flow analysis
* Material balance planning
*Fluid mechanics
Fluid mechanics is the branch of physics concerned with the mechanics of fluids (liquids, gases, and plasma (physics), plasmas) and the forces on them.
Originally applied to water (hydromechanics), it found applications in a wide range of discipl ...
References
External links
Material Balance Calculations
Material Balance Fundamentals
The Material Balance for Chemical Reactors
Material and energy balance
* {{cite book , last = Morris , first = Arthur E. , last2 = Geiger , first2 = Gordon , last3 = Fine , first3 = H. Alan , title = Handbook on Material and Energy Balance Calculations in Material Processing , url = http://www.wiley.com/WileyCDA/WileyTitle/productCd-1118065654.html , edition = 3rd , date = 2011 , isbn = 978-1-118-06565-5 , publisher = Wiley Mass Chemical process engineering Transport phenomena