Marine Biogeochemical Cycles on:
[Wikipedia]
[Google]
[Amazon]
Marine biogeochemical cycles are
''OpenStax'', 9 May 2019. Modified text was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
The six aforementioned elements are used by organisms in a variety of ways. Hydrogen and oxygen are found in water and organic molecules, both of which are essential to life. Carbon is found in all organic molecules, whereas nitrogen is an important component of nucleic acids and proteins. Phosphorus is used to make nucleic acids and the phospholipids that comprise biological membranes. Sulfur is critical to the three-dimensional shape of proteins. The cycling of these elements is interconnected. For example, the movement of water is critical for leaching sulfur and phosphorus into rivers which can then flow into oceans. Minerals cycle through the biosphere between the biotic and abiotic components and from one organism to another.Fisher M. R. (Ed.) (2019) ''Environmental Biology''
3.2 Biogeochemical Cycles
OpenStax. Modified text was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
 Water is the medium of the oceans, the medium which carries all the substances and elements involved in the marine biogeochemical cycles.
Water as found in nature almost always includes dissolved substances, so water has been described as the "universal solvent" for its ability to dissolve so many substances. This ability allows it to be the "
Water is the medium of the oceans, the medium which carries all the substances and elements involved in the marine biogeochemical cycles.
Water as found in nature almost always includes dissolved substances, so water has been described as the "universal solvent" for its ability to dissolve so many substances. This ability allows it to be the "
''NOAA''. Last updated: 26 February 2021. Evaporation of ocean water and formation of sea ice further increase the salinity of the ocean. However these processes which increase salinity are continually counterbalanced by processes that decrease salinity, such as the continuous input of fresh water from rivers, precipitation of rain and snow, and the melting of ice.Salinity
''NASA''. Last updated: 7 April 2021. The two most prevalent ions in seawater are chloride and sodium. Together, they make up around 85 per cent of all dissolved ions in the ocean. Magnesium and sulfate ions make up most of the rest. Salinity varies with temperature, evaporation, and precipitation. It is generally low at the equator and poles, and high at mid-latitudes.
File:SeaSurfaceSalinity.jpg, Annual mean sea surface salinity, measured in 2009 in practical salinity units
File:Vertical differences in ocean salinity (0 to 300m).png, Vertical differences in sea salinity between the surface and a depth of 300 metres. Salinity increases with depth in red regions and decreases in blue regions. Modified text was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
 A stream of airborne microorganisms circles the planet above weather systems but below commercial air lanes. Some peripatetic microorganisms are swept up from terrestrial dust storms, but most originate from marine microorganisms in
A stream of airborne microorganisms circles the planet above weather systems but below commercial air lanes. Some peripatetic microorganisms are swept up from terrestrial dust storms, but most originate from marine microorganisms in

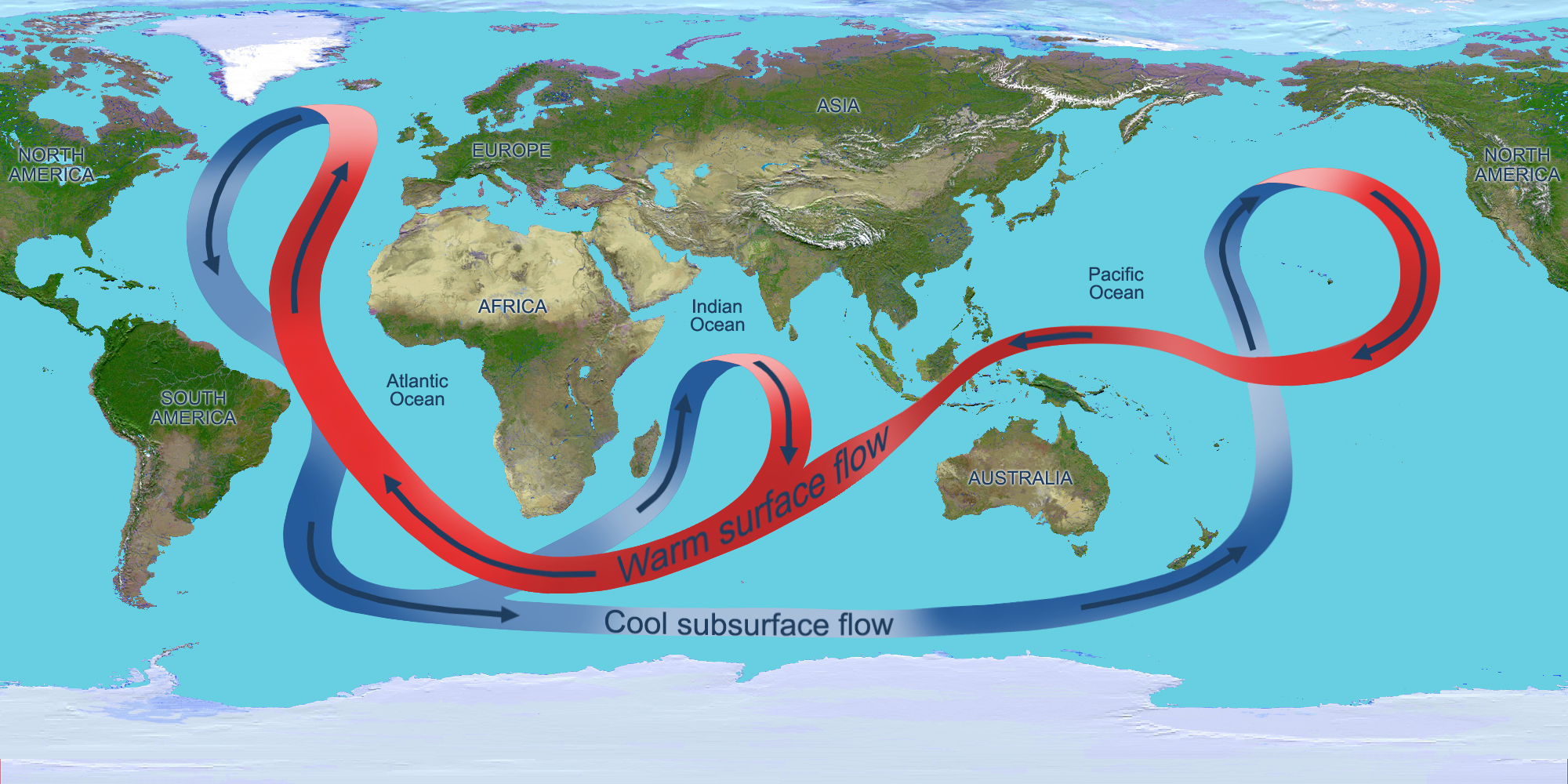 Solar radiation affects the oceans: warm water from the Equator tends to circulate toward the poles, while cold polar water heads towards the Equator. The surface currents are initially dictated by surface wind conditions. The
Solar radiation affects the oceans: warm water from the Equator tends to circulate toward the poles, while cold polar water heads towards the Equator. The surface currents are initially dictated by surface wind conditions. The
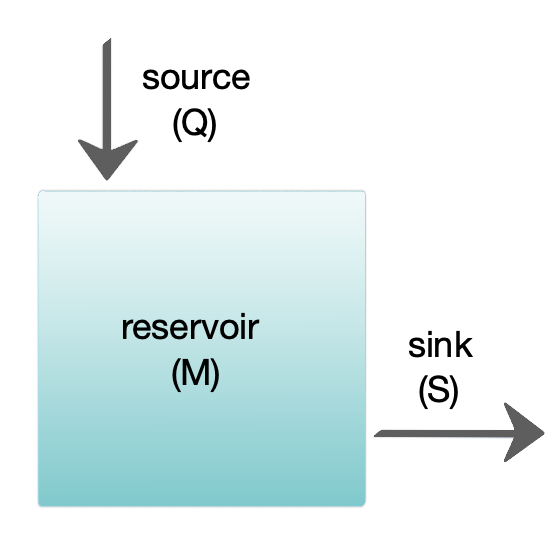 Box models are widely used to model biogeochemical systems. Box models are simplified versions of complex systems, reducing them to boxes (or storage
Box models are widely used to model biogeochemical systems. Box models are simplified versions of complex systems, reducing them to boxes (or storage 
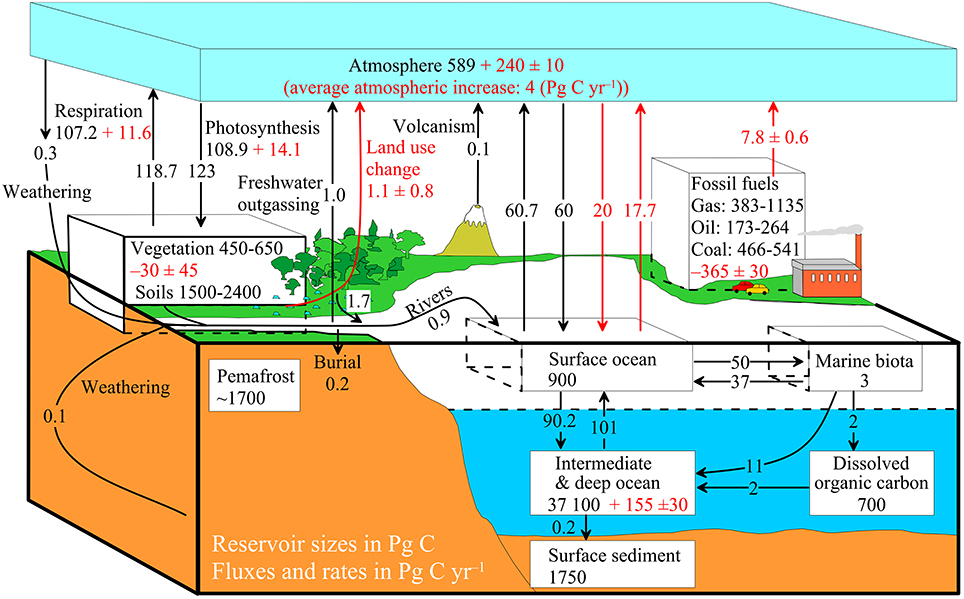 The diagram above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the
The diagram above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the
 The nitrogen cycle is as important in the ocean as it is on land. While the overall cycle is similar in both cases, there are different players and modes of transfer for nitrogen in the ocean. Nitrogen enters the ocean through precipitation, runoff, or as N2 from the atmosphere. Nitrogen cannot be utilized by
The nitrogen cycle is as important in the ocean as it is on land. While the overall cycle is similar in both cases, there are different players and modes of transfer for nitrogen in the ocean. Nitrogen enters the ocean through precipitation, runoff, or as N2 from the atmosphere. Nitrogen cannot be utilized by
 A
A
''Earth in the Future'', PenState/NASSA. Retrieved 18 June 2020. The process of cycling nutrients in the sea starts with biological pumping, when nutrients are extracted from surface waters by phytoplankton to become part of their organic makeup. Phytoplankton are either eaten by other organisms, or eventually die and drift down as
 Sulfate reduction in the seabed is strongly focused toward near-surface sediments with high depositional rates along the ocean margins. The benthic marine sulfur cycle is therefore sensitive to anthropogenic influence, such as ocean warming and increased nutrient loading of coastal seas. This stimulates photosynthetic productivity and results in enhanced export of organic matter to the seafloor, often combined with low oxygen concentration in the bottom water (Rabalais et al., 2014; Breitburg et al., 2018). The biogeochemical zonation is thereby compressed toward the sediment surface, and the balance of organic matter mineralization is shifted from oxic and suboxic processes toward sulfate reduction and methanogenesis (Middelburg and Levin, 2009).
*
Sulfate reduction in the seabed is strongly focused toward near-surface sediments with high depositional rates along the ocean margins. The benthic marine sulfur cycle is therefore sensitive to anthropogenic influence, such as ocean warming and increased nutrient loading of coastal seas. This stimulates photosynthetic productivity and results in enhanced export of organic matter to the seafloor, often combined with low oxygen concentration in the bottom water (Rabalais et al., 2014; Breitburg et al., 2018). The biogeochemical zonation is thereby compressed toward the sediment surface, and the balance of organic matter mineralization is shifted from oxic and suboxic processes toward sulfate reduction and methanogenesis (Middelburg and Levin, 2009).
*
 The
The  Iron is an essential micronutrient for almost every life form. It is a key component of hemoglobin, important to nitrogen fixation as part of the
Iron is an essential micronutrient for almost every life form. It is a key component of hemoglobin, important to nitrogen fixation as part of the

 With its close relation to the
With its close relation to the 
 "Biological activity is a dominant force shaping the chemical structure and evolution of the earth surface environment. The presence of an oxygenated atmosphere-hydrosphere surrounding an otherwise highly reducing solid earth is the most striking consequence of the rise of life on earth. Biological evolution and the functioning of ecosystems, in turn, are to a large degree conditioned by geophysical and geological processes. Understanding the interactions between organisms and their abiotic environment, and the resulting coupled evolution of the biosphere and geosphere is a central theme of research in biogeology. Biogeochemists contribute to this understanding by studying the transformations and transport of chemical substrates and products of biological activity in the environment."
"Since the Cambrian explosion, mineralized body parts have been secreted in large quantities by biota. Because calcium carbonate, silica and calcium phosphate are the main mineral phases constituting these hard parts, biomineralization plays an important role in the global biogeochemical cycles of carbon, calcium, silicon and phosphorus"
"Biological activity is a dominant force shaping the chemical structure and evolution of the earth surface environment. The presence of an oxygenated atmosphere-hydrosphere surrounding an otherwise highly reducing solid earth is the most striking consequence of the rise of life on earth. Biological evolution and the functioning of ecosystems, in turn, are to a large degree conditioned by geophysical and geological processes. Understanding the interactions between organisms and their abiotic environment, and the resulting coupled evolution of the biosphere and geosphere is a central theme of research in biogeology. Biogeochemists contribute to this understanding by studying the transformations and transport of chemical substrates and products of biological activity in the environment."
"Since the Cambrian explosion, mineralized body parts have been secreted in large quantities by biota. Because calcium carbonate, silica and calcium phosphate are the main mineral phases constituting these hard parts, biomineralization plays an important role in the global biogeochemical cycles of carbon, calcium, silicon and phosphorus"
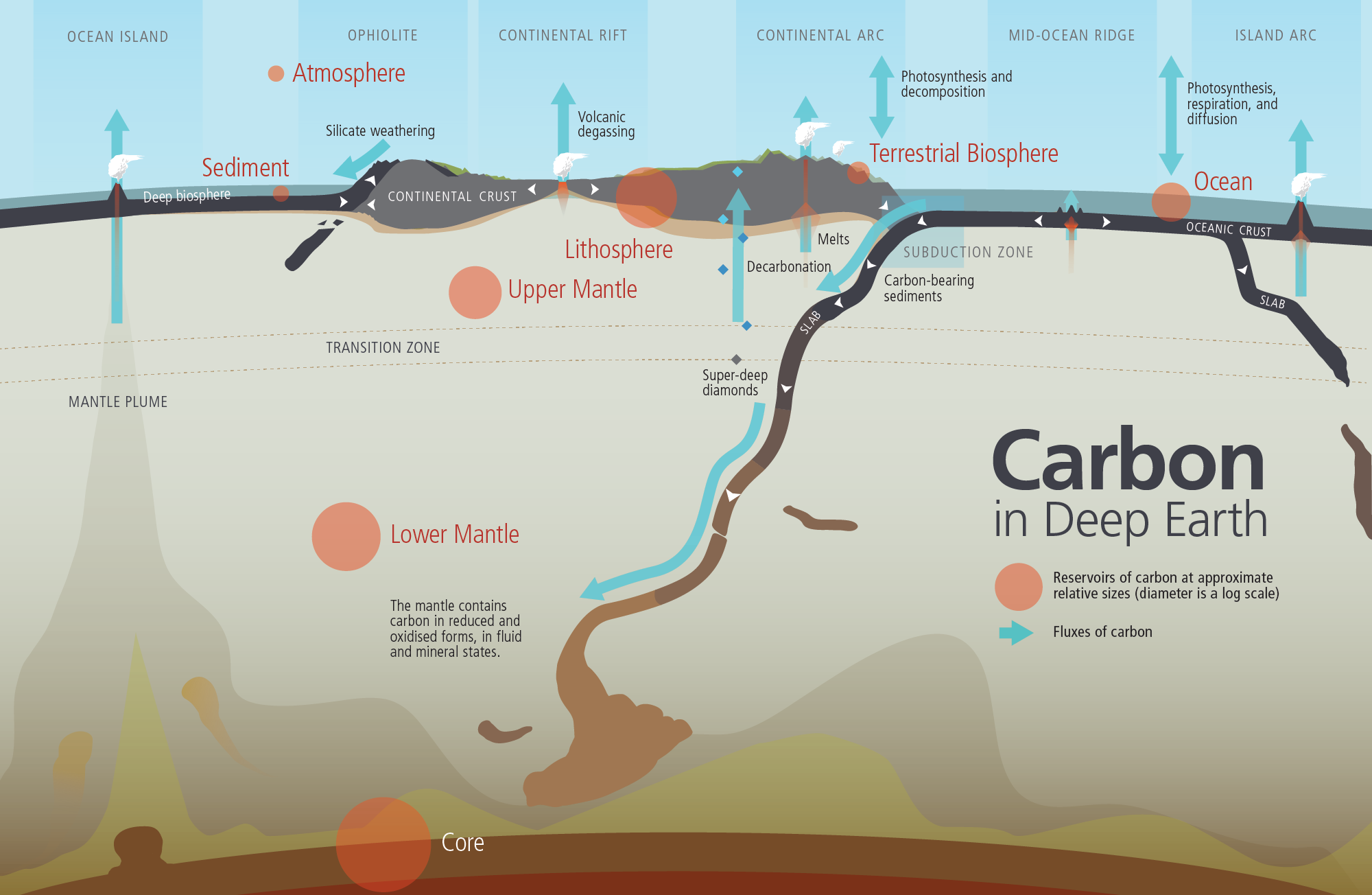 Deep cycling involves the exchange of materials with the mantle. The deep water cycle involves exchange of water with the mantle, with water carried down by
Deep cycling involves the exchange of materials with the mantle. The deep water cycle involves exchange of water with the mantle, with water carried down by

''Giant Oil and Gas Fields of the Decade, 1990–1999''
Tulsa, Okla.:
File:Lead Cycle.jpg,
File:MercuryFoodChain.svg,
biogeochemical cycle
A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cyc ...
s that occur within marine environments, that is, in the saltwater of seas or oceans or the brackish
Brackish water, sometimes termed brack water, is water occurring in a natural environment that has more salinity than freshwater, but not as much as seawater. It may result from mixing seawater (salt water) and fresh water together, as in estuari ...
water of coastal estuaries
An estuary is a partially enclosed coastal body of brackish water with one or more rivers or streams flowing into it, and with a free connection to the open sea. Estuaries form a transition zone between river environments and maritime environm ...
. These biogeochemical cycles are the pathways chemical substance
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be com ...
s and elements move through within the marine environment. In addition, substances and elements can be imported into or exported from the marine environment. These imports and exports can occur as exchanges with the atmosphere above, the ocean floor below, or as runoff from the land.
There are biogeochemical
Biogeochemistry is the scientific discipline that involves the study of the chemical, physical, geological, and biological processes and reactions that govern the composition of the natural environment (including the biosphere, the cryosphere, ...
cycles for the elements calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
, carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, mercury, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
, selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ...
, and sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
; molecular cycles for water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
and silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
; macroscopic cycles such as the rock cycle
The ''rock cycle'' is a basic concept in geology that describes transitions through geologic time among the three main rock types: sedimentary, metamorphic, and igneous. Each rock type is altered when it is forced out of its equilibrium cond ...
; as well as human-induced cycles for synthetic compounds such as polychlorinated biphenyl
Polychlorinated biphenyls (PCBs) are organochlorine compounds with the formula Carbon, C12Hydrogen, H10−''x''Chloride, Cl''x''; they were once widely used in the manufacture of carbonless copy paper, as heat transfer fluids, and as dielectri ...
(PCB). In some cycles there are reservoirs where a substance can be stored for a long time. The cycling of these elements is interconnected.
Marine organisms
Marine life, sea life or ocean life is the collective ecological communities that encompass all aquatic animals, plants, algae, fungi, protists, single-celled microorganisms and associated viruses living in the saline water of marine habita ...
, and particularly marine microorganisms
Marine microorganisms are defined by their habitat as microorganisms living in a marine habitat, marine environment, that is, in the saline water, saltwater of a sea or ocean or the brackish water of a coastal estuary. A microorganism (or mic ...
are crucial for the functioning of many of these cycles. The forces driving biogeochemical cycles include metabolic processes within organisms, geological processes involving the Earth's mantle, as well as chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
s among the substances themselves, which is why these are called biogeochemical cycles. While chemical substances can be broken down and recombined, the chemical elements themselves can be neither created nor destroyed by these forces, so apart from some losses to and gains from outer space, elements are recycled or stored (sequestered) somewhere on or within the planet.
Overview
Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their environment is called a biogeochemical cycle.Biogeochemical Cycles''OpenStax'', 9 May 2019. Modified text was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
The six aforementioned elements are used by organisms in a variety of ways. Hydrogen and oxygen are found in water and organic molecules, both of which are essential to life. Carbon is found in all organic molecules, whereas nitrogen is an important component of nucleic acids and proteins. Phosphorus is used to make nucleic acids and the phospholipids that comprise biological membranes. Sulfur is critical to the three-dimensional shape of proteins. The cycling of these elements is interconnected. For example, the movement of water is critical for leaching sulfur and phosphorus into rivers which can then flow into oceans. Minerals cycle through the biosphere between the biotic and abiotic components and from one organism to another.Fisher M. R. (Ed.) (2019) ''Environmental Biology''
3.2 Biogeochemical Cycles
OpenStax. Modified text was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
The water cycle
 Water is the medium of the oceans, the medium which carries all the substances and elements involved in the marine biogeochemical cycles.
Water as found in nature almost always includes dissolved substances, so water has been described as the "universal solvent" for its ability to dissolve so many substances. This ability allows it to be the "
Water is the medium of the oceans, the medium which carries all the substances and elements involved in the marine biogeochemical cycles.
Water as found in nature almost always includes dissolved substances, so water has been described as the "universal solvent" for its ability to dissolve so many substances. This ability allows it to be the "solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
of life" Water is also the only common substance that exists as solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
, liquid, and gas
Gas is a state of matter that has neither a fixed volume nor a fixed shape and is a compressible fluid. A ''pure gas'' is made up of individual atoms (e.g. a noble gas like neon) or molecules of either a single type of atom ( elements such as ...
in normal terrestrial conditions. Since liquid water flows, ocean waters cycle and flow in currents around the world. Since water easily changes phase, it can be carried into the atmosphere as water vapour or frozen as an iceberg. It can then precipitate or melt to become liquid water again. All marine life is immersed in water, the matrix and womb of life itself. Water can be broken down into its constituent hydrogen and oxygen by metabolic or abiotic processes, and later recombined to become water again.
While the water cycle is itself a biogeochemical cycle
A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cyc ...
, flow of water over and beneath the Earth is a key component of the cycling of other biogeochemicals. Runoff is responsible for almost all of the transport of eroded
Erosion is the action of surface processes (such as water flow or wind) that removes soil, rock, or dissolved material from one location on the Earth's crust and then transports it to another location where it is deposited. Erosion is disti ...
sediment
Sediment is a solid material that is transported to a new location where it is deposited. It occurs naturally and, through the processes of weathering and erosion, is broken down and subsequently sediment transport, transported by the action of ...
and phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
from land to waterbodies
A body of water or waterbody is any significant accumulation of water on the surface of Earth or another planet. The term most often refers to oceans, seas, and lakes, but it includes smaller pools of water such as ponds, wetlands, or more ra ...
. Cultural eutrophication
Eutrophication is a general term describing a process in which nutrients accumulate in a body of water, resulting in an increased growth of organisms that may deplete the oxygen in the water; ie. the process of too many plants growing on the s ...
of lakes is primarily due to phosphorus, applied in excess to agricultural fields in fertilizer
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Man ...
s, and then transported overland and down rivers. Both runoff and groundwater flow play significant roles in transporting nitrogen from the land to waterbodies. The dead zone
Dead zone may refer to:
Science and technology
* Dead zone (cell phone), an area where cell phones cannot transmit to a nearby cell site
* Dead zone (ecology), low-oxygen areas in the world's oceans
* Dead band, the region of insensitivity of a ...
at the outlet of the Mississippi River
The Mississippi River is the main stem, primary river of the largest drainage basin in the United States. It is the second-longest river in the United States, behind only the Missouri River, Missouri. From its traditional source of Lake Ita ...
is a consequence of nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
s from fertilizer being carried off agricultural fields and funnelled down the river system
In geomorphology, drainage systems, also known as river systems, are the patterns formed by the streams, rivers, and lakes in a particular drainage basin. They are governed by the topography of land, whether a particular region is dominated by har ...
to the Gulf of Mexico
The Gulf of Mexico () is an oceanic basin and a marginal sea of the Atlantic Ocean, mostly surrounded by the North American continent. It is bounded on the northeast, north, and northwest by the Gulf Coast of the United States; on the southw ...
. Runoff also plays a part in the carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
, again through the transport of eroded rock and soil.
Ocean salinity
Ocean salinity is derived mainly from the weathering of rocks and the transport of dissolved salts from the land, with lesser contributions fromhydrothermal vent
Hydrothermal vents are fissures on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hot ...
s in the seafloor.Why is the ocean salty?''NOAA''. Last updated: 26 February 2021. Evaporation of ocean water and formation of sea ice further increase the salinity of the ocean. However these processes which increase salinity are continually counterbalanced by processes that decrease salinity, such as the continuous input of fresh water from rivers, precipitation of rain and snow, and the melting of ice.Salinity
''NASA''. Last updated: 7 April 2021. The two most prevalent ions in seawater are chloride and sodium. Together, they make up around 85 per cent of all dissolved ions in the ocean. Magnesium and sulfate ions make up most of the rest. Salinity varies with temperature, evaporation, and precipitation. It is generally low at the equator and poles, and high at mid-latitudes.
Creative Commons Attribution 4.0 International License
Sea spray
 A stream of airborne microorganisms circles the planet above weather systems but below commercial air lanes. Some peripatetic microorganisms are swept up from terrestrial dust storms, but most originate from marine microorganisms in
A stream of airborne microorganisms circles the planet above weather systems but below commercial air lanes. Some peripatetic microorganisms are swept up from terrestrial dust storms, but most originate from marine microorganisms in sea spray
Sea spray consists of aerosol particles formed from the ocean, primarily by ejection into Earth's atmosphere through bursting bubbles at the air-sea interface Sea spray contains both organic matter and inorganic salts that form sea salt aeroso ...
. In 2018, scientists reported that hundreds of millions of viruses and tens of millions of bacteria are deposited daily on every square meter around the planet. This is another example of water facilitating the transport of organic material over great distances, in this case in the form of live microorganisms.
Dissolved salt does not evaporate back into the atmosphere like water, but it does form sea salt aerosols in sea spray
Sea spray consists of aerosol particles formed from the ocean, primarily by ejection into Earth's atmosphere through bursting bubbles at the air-sea interface Sea spray contains both organic matter and inorganic salts that form sea salt aeroso ...
. Many physical process
Physical changes are changes affecting the form of a chemical substance, but not its chemical composition. Physical changes are used to separate mixtures into their component compounds, but can not usually be used to separate compounds into chem ...
es over ocean surface generate sea salt aerosols. One common cause is the bursting of air bubbles, which are entrained by the wind stress during the whitecap formation. Another is tearing of drops from wave tops. The total sea salt flux from the ocean to the atmosphere is about 3300 Tg (3.3 billion tonnes) per year.
Ocean circulation

 Solar radiation affects the oceans: warm water from the Equator tends to circulate toward the poles, while cold polar water heads towards the Equator. The surface currents are initially dictated by surface wind conditions. The
Solar radiation affects the oceans: warm water from the Equator tends to circulate toward the poles, while cold polar water heads towards the Equator. The surface currents are initially dictated by surface wind conditions. The trade winds
The trade winds or easterlies are permanent east-to-west prevailing winds that flow in the Earth's equatorial region. The trade winds blow mainly from the northeast in the Northern Hemisphere and from the southeast in the Southern Hemisphere ...
blow westward in the tropics, and the westerlies
The westerlies, anti-trades, or prevailing westerlies, are prevailing winds from the west toward the east in the middle latitudes between 30 and 60 degrees latitude. They originate from the high-pressure areas in the horse latitudes (about ...
blow eastward at mid-latitudes. This wind pattern applies a stress to the subtropical ocean surface with negative curl
cURL (pronounced like "curl", ) is a free and open source computer program for transferring data to and from Internet servers. It can download a URL from a web server over HTTP, and supports a variety of other network protocols, URI scheme ...
across the Northern Hemisphere
The Northern Hemisphere is the half of Earth that is north of the equator. For other planets in the Solar System, north is defined by humans as being in the same celestial sphere, celestial hemisphere relative to the invariable plane of the Solar ...
, and the reverse across the Southern Hemisphere. The resulting Sverdrup transport
The Sverdrup balance, or Sverdrup relation, is a theoretical relationship between the wind stress exerted on the surface of the open ocean and the vertically integrated meridional (north-south) transport of ocean water.
History
Aside from the ...
is equatorward. Because of conservation of potential vorticity
In fluid mechanics, potential vorticity (PV) is a quantity which is proportional to the dot product of vorticity and stratification. This quantity, following a parcel of air or water, can only be changed by diabatic or frictional processes. I ...
caused by the poleward-moving winds on the subtropical ridge
The horse latitudes are the latitudes about 30 degrees north and south of the Equator. They are characterized by sunny skies, calm winds, and very little precipitation. They are also known as subtropical ridges or highs. It is a high-pressur ...
's western periphery and the increased relative vorticity of poleward moving water, transport is balanced by a narrow, accelerating poleward current, which flows along the western boundary of the ocean basin, outweighing the effects of friction with the cold western boundary current which originates from high latitudes. The overall process, known as western intensification, causes currents on the western boundary of an ocean basin to be stronger than those on the eastern boundary.
As it travels poleward, warm water transported by strong warm water current undergoes evaporative cooling. The cooling is wind driven: wind moving over water cools the water and also causes evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evapora ...
, leaving a saltier brine. In this process, the water becomes saltier and denser. and decreases in temperature. Once sea ice forms, salts are left out of the ice, a process known as brine exclusion. These two processes produce water that is denser and colder. The water across the northern Atlantic Ocean
The Atlantic Ocean is the second largest of the world's five borders of the oceans, oceanic divisions, with an area of about . It covers approximately 17% of Earth#Surface, Earth's surface and about 24% of its water surface area. During the ...
becomes so dense that it begins to sink down through less salty and less dense water. This downdraft of heavy, cold and dense water becomes a part of the North Atlantic Deep Water
North Atlantic Deep Water (NADW) is a deep water mass formed in the North Atlantic Ocean. Thermohaline circulation (properly described as meridional overturning circulation) of the world's oceans involves the flow of warm surface waters from the ...
, a southgoing stream.
Winds drive ocean currents in the upper 100 meters of the ocean's surface. However, ocean currents also flow thousands of meters below the surface. These deep-ocean currents are driven by differences in the water's density, which is controlled by temperature (thermo) and salinity (haline). This process is known as thermohaline circulation. In the Earth's polar regions ocean water gets very cold, forming sea ice. As a consequence the surrounding seawater gets saltier, because when sea ice forms, the salt is left behind. As the seawater gets saltier, its density increases, and it starts to sink. Surface water is pulled in to replace the sinking water, which in turn eventually becomes cold and salty enough to sink. This initiates the deep-ocean currents driving the global conveyor belt.
Thermohaline circulation drives a global-scale system of currents called the “global conveyor belt.” The conveyor belt begins on the surface of the ocean near the pole in the North Atlantic. Here, the water is chilled by Arctic temperatures. It also gets saltier because when sea ice forms, the salt does not freeze and is left behind in the surrounding water. The cold water is now more dense, due to the added salts, and sinks toward the ocean bottom. Surface water moves in to replace the sinking water, thus creating a current. This deep water moves south, between the continents, past the equator, and down to the ends of Africa and South America. The current travels around the edge of Antarctica, where the water cools and sinks again, as it does in the North Atlantic. Thus, the conveyor belt gets "recharged." As it moves around Antarctica, two sections split off the conveyor and turn northward. One section moves into the Indian Ocean, the other into the Pacific Ocean. These two sections that split off warm up and become less dense as they travel northward toward the equator, so that they rise to the surface (upwelling). They then loop back southward and westward to the South Atlantic, eventually returning to the North Atlantic, where the cycle begins again. The conveyor belt moves at much slower speeds (a few centimeters per second) than wind-driven or tidal currents (tens to hundreds of centimeters per second). It is estimated that any given cubic meter of water takes about 1,000 years to complete the journey along the global conveyor belt. In addition, the conveyor moves an immense volume of water—more than 100 times the flow of the Amazon River (Ross, 1995). The conveyor belt is also a vital component of the global ocean nutrient and carbon dioxide cycles. Warm surface waters are depleted of nutrients and carbon dioxide, but they are enriched again as they travel through the conveyor belt as deep or bottom layers. The base of the world's food chain depends on the cool, nutrient-rich waters that support the growth of algae and seaweed.
The global average residence time of a water molecule in the ocean is about 3,200 years. By comparison the average residence time in the atmosphere is about nine days. If it is frozen in the Antarctic or drawn into deep groundwater it can be sequestered for ten thousand years.
Cycling of key elements
Box models
 Box models are widely used to model biogeochemical systems. Box models are simplified versions of complex systems, reducing them to boxes (or storage
Box models are widely used to model biogeochemical systems. Box models are simplified versions of complex systems, reducing them to boxes (or storage reservoir
A reservoir (; ) is an enlarged lake behind a dam, usually built to water storage, store fresh water, often doubling for hydroelectric power generation.
Reservoirs are created by controlling a watercourse that drains an existing body of wa ...
s) for chemical materials, linked by material flux
Flux describes any effect that appears to pass or travel (whether it actually moves or not) through a surface or substance. Flux is a concept in applied mathematics and vector calculus which has many applications in physics. For transport phe ...
es (flows). Simple box models have a small number of boxes with properties, such as volume, that do not change with time. The boxes are assumed to behave as if they were mixed homogeneously. These models are often used to derive analytical formulas describing the dynamics and steady-state abundance of the chemical species involved.
The diagram at the right shows a basic one-box model. The reservoir contains the amount of material ''M'' under consideration, as defined by chemical, physical or biological properties. The source ''Q'' is the flux of material into the reservoir, and the sink ''S'' is the flux of material out of the reservoir. The budget is the check and balance of the sources and sinks affecting material turnover in a reservoir. The reservoir is in a steady state
In systems theory, a system or a process is in a steady state if the variables (called state variables) which define the behavior of the system or the process are unchanging in time. In continuous time, this means that for those properties ''p' ...
if ''Q'' = ''S'', that is, if the sources balance the sinks and there is no change over time.
The turnover time (also called the renewal time or exit age) is the average time material spends resident in the reservoir. If the reservoir is in a steady state, this is the same as the time it takes to fill or drain the reservoir. Thus, if τ is the turnover time, then τ = M/S. The equation describing the rate of change of content in a reservoir is
::
When two or more reservoirs are connected, the material can be regarded as cycling between the reservoirs, and there can be predictable patterns to the cyclic flow. More complex multibox models are usually solved using numerical techniques.

 The diagram above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the
The diagram above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the euphotic zone
The photic zone (or euphotic zone, epipelagic zone, or sunlight zone) is the uppermost layer of a body of water that receives sunlight, allowing phytoplankton to perform photosynthesis. It undergoes a series of physical, chemical, and biological ...
, one for the ocean interior or dark ocean, and one for ocean sediment
Marine sediment, or ocean sediment, or seafloor sediment, are deposits of insoluble particles that have accumulated on the seafloor. These particles either have their origins in soil and rocks and have been transported from the land to the ...
s. In the euphotic zone, net phytoplankton production is about 50 Pg C each year. About 10 Pg is exported to the ocean interior while the other 40 Pg is respired. Organic carbon degradation occurs as particles
In the physical sciences, a particle (or corpuscle in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
(marine snow
In the deep ocean, marine snow (also known as "ocean dandruff") is a continuous shower of mostly organic detritus falling from the upper layers of the water column. It is a significant means of exporting energy from the light-rich photic zone to ...
) settle through the ocean interior. Only 2 Pg eventually arrives at the seafloor, while the other 8 Pg is respired in the dark ocean. In sediments, the time scale available for degradation increases by orders of magnitude with the result that 90% of the organic carbon delivered is degraded and only 0.2 Pg C yr−1 is eventually buried and transferred from the biosphere to the geosphere.
Dissolved and particulate matter
Biological pumps
Thebiological pump
The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven Carbon sequestration, sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sedim ...
, in its simplest form, is the ocean's biologically driven sequestration of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
from the atmosphere to the ocean interior and seafloor sediments. It is the part of the oceanic carbon cycle
The oceanic carbon cycle (or marine carbon cycle) is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many inter ...
responsible for the cycling of organic matter
Organic matter, organic material or natural organic matter is the large source of carbon-based compounds found within natural and engineered, terrestrial, and aquatic environments. It is matter composed of organic compounds that have come fro ...
formed mainly by phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
during photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
(soft-tissue pump), as well as the cycling of calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
(CaCO3) formed into shells by certain organisms such as plankton
Plankton are the diverse collection of organisms that drift in Hydrosphere, water (or atmosphere, air) but are unable to actively propel themselves against ocean current, currents (or wind). The individual organisms constituting plankton are ca ...
and mollusks
Mollusca is a phylum of protostomic invertebrate animals, whose members are known as molluscs or mollusks (). Around 76,000 extant species of molluscs are recognized, making it the second-largest animal phylum after Arthropoda. The num ...
(carbonate pump).
The biological pump can be divided into three distinct phases,De La Rocha CL. 2006. The Biological Pump. In: Treatise on Geochemistry; vol. 6, (ed.). Pergamon Press, pp. 83-111 the first of which is the production of fixed carbon by planktonic phototrophs in the euphotic
The photic zone (or euphotic zone, epipelagic zone, or sunlight zone) is the uppermost layer of a body of water that receives sunlight, allowing phytoplankton to perform photosynthesis. It undergoes a series of physical, chemical, and biological ...
(sunlit) surface region of the ocean. In these surface waters, phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
use carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
(CO2), nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
(N), phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
(P), and other trace elements (barium
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
, iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
, etc.) during photosynthesis to make carbohydrates
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ma ...
, lipids
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins Vitamin A, A, Vitamin D, D, Vitamin E, E and Vitamin K, K), monoglycerides, diglycerides, phospholipids, and others. The fu ...
, and proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, re ...
. Some plankton, (e.g. coccolithophores
Coccolithophores, or coccolithophorids, are single-celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingd ...
and foraminifera
Foraminifera ( ; Latin for "hole bearers"; informally called "forams") are unicellular organism, single-celled organisms, members of a phylum or class (biology), class of Rhizarian protists characterized by streaming granular Ectoplasm (cell bio ...
) combine calcium (Ca) and dissolved carbonates (carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
and bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
) to form a calcium carbonate (CaCO3) protective coating.
Once this carbon is fixed into soft or hard tissue, the organisms either stay in the euphotic zone to be recycled as part of the regenerative nutrient cycle
A nutrient cycle (or ecological recycling) is the movement and exchange of inorganic and organic matter back into the production of matter. Energy flow is a unidirectional and noncyclic pathway, whereas the movement of mineral nutrients is cyc ...
or once they die, continue to the second phase of the biological pump and begin to sink to the ocean floor. The sinking particles will often form aggregates as they sink, greatly increasing the sinking rate. It is this aggregation that gives particles a better chance of escaping predation and decomposition in the water column and eventually make it to the sea floor.
The fixed carbon that is either decomposed by bacteria on the way down or once on the sea floor then enters the final phase of the pump and is remineralized to be used again in primary production
In ecology, primary production is the synthesis of organic compounds from atmospheric or aqueous carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through ...
. The particles that escape these processes entirely are sequestered in the sediment and may remain there for millions of years. It is this sequestered carbon that is responsible for ultimately lowering atmospheric CO2.
* Brum JR, Morris JJ, Décima M and Stukel MR (2014) "Mortality in the oceans: Causes and consequences". ''Eco-DAS IX Symposium Proceedings'', Chapter 2, pages 16–48. Association for the Sciences of Limnology and Oceanography. .
*
*
Role of microorganisms
Carbon, oxygen and hydrogen cycles
Themarine carbon cycle
The oceanic carbon cycle (or marine carbon cycle) is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the Seabed, seafloor. The carbon cycle is a result of ma ...
is composed of processes that exchange carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor
The seabed (also known as the seafloor, sea floor, ocean floor, and ocean bottom) is the bottom of the ocean. All floors of the ocean are known as seabeds.
The structure of the seabed of the global ocean is governed by plate tectonics. Most of ...
. The carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''.
Inor ...
carbon (carbon not associated with a living thing, such as carbon dioxide) and organic carbon (carbon that is, or has been, incorporated into a living thing). Part of the marine carbon cycle transforms carbon between non-living and living matter.
Three main processes (or pumps) that make up the marine carbon cycle bring atmospheric carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
(CO2) into the ocean interior and distribute it through the oceans. These three pumps are: (1) the solubility pump, (2) the carbonate pump, and (3) the biological pump. The total active pool of carbon at the Earth's surface for durations of less than 10,000 years is roughly 40,000 gigatons C (Gt C, a gigaton is one billion tons, or the weight of approximately 6 million blue whale
The blue whale (''Balaenoptera musculus'') is a marine mammal and a baleen whale. Reaching a maximum confirmed length of and weighing up to , it is the largest animal known ever to have existed. The blue whale's long and slender body can ...
s), and about 95% (~38,000 Gt C) is stored in the ocean, mostly as dissolved inorganic carbon. The speciation
Speciation is the evolutionary process by which populations evolve to become distinct species. The biologist Orator F. Cook coined the term in 1906 for cladogenesis, the splitting of lineages, as opposed to anagenesis, phyletic evolution within ...
of dissolved inorganic carbon in the marine carbon cycle is a primary controller of acid-base chemistry in the oceans.
Nitrogen and phosphorus cycles
 The nitrogen cycle is as important in the ocean as it is on land. While the overall cycle is similar in both cases, there are different players and modes of transfer for nitrogen in the ocean. Nitrogen enters the ocean through precipitation, runoff, or as N2 from the atmosphere. Nitrogen cannot be utilized by
The nitrogen cycle is as important in the ocean as it is on land. While the overall cycle is similar in both cases, there are different players and modes of transfer for nitrogen in the ocean. Nitrogen enters the ocean through precipitation, runoff, or as N2 from the atmosphere. Nitrogen cannot be utilized by phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
as N2 so it must undergo nitrogen fixation
Nitrogen fixation is a chemical process by which molecular dinitrogen () is converted into ammonia (). It occurs both biologically and abiological nitrogen fixation, abiologically in chemical industry, chemical industries. Biological nitrogen ...
which is performed predominantly by cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteri ...
. Without supplies of fixed nitrogen entering the marine cycle, the fixed nitrogen would be used up in about 2000 years. Phytoplankton need nitrogen in biologically available forms for the initial synthesis of organic matter. Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
and urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
are released into the water by excretion from plankton. Nitrogen sources are removed from the euphotic zone
The photic zone (or euphotic zone, epipelagic zone, or sunlight zone) is the uppermost layer of a body of water that receives sunlight, allowing phytoplankton to perform photosynthesis. It undergoes a series of physical, chemical, and biological ...
by the downward movement of the organic matter. This can occur from sinking of phytoplankton, vertical mixing, or sinking of waste of vertical migrators. The sinking results in ammonia being introduced at lower depths below the euphotic zone. Bacteria are able to convert ammonia to nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
and nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
but they are inhibited by light so this must occur below the euphotic zone. Ammonification or mineralization is performed by bacteria to convert organic nitrogen to ammonia. Nitrification
''Nitrification'' is the biological oxidation of ammonia to nitrate via the intermediary nitrite. Nitrification is an important step in the nitrogen cycle in soil. The process of complete nitrification may occur through separate organisms or ent ...
can then occur to convert the ammonium to nitrite and nitrate. Nitrate can be returned to the euphotic zone by vertical mixing and upwelling
Upwelling is an physical oceanography, oceanographic phenomenon that involves wind-driven motion of dense, cooler, and usually nutrient-rich water from deep water towards the ocean surface. It replaces the warmer and usually nutrient-depleted sur ...
where it can be taken up by phytoplankton to continue the cycle. N2 can be returned to the atmosphere through denitrification
Denitrification is a microbially facilitated process where nitrate (NO3−) is reduced and ultimately produces molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. Facultative anaerobic bacteria perform denitr ...
.
Ammonium is thought to be the preferred source of fixed nitrogen for phytoplankton because its assimilation does not involve a redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
reaction and therefore requires little energy. Nitrate requires a redox reaction for assimilation but is more abundant so most phytoplankton have adapted to have the enzymes necessary to undertake this reduction (nitrate reductase
Nitrate reductases are molybdoenzymes that reduce nitrate () to nitrite (). This reaction is critical for the production of protein in most crop plants, as nitrate is the predominant source of nitrogen in fertilized soils.
Types
Euka ...
). There are a few notable and well-known exceptions that include most ''Prochlorococcus
''Prochlorococcus'' is a genus of very small (0.6 μm) marine cyanobacteria with an unusual pigmentation ( chlorophyll ''a2'' and ''b2''). These bacteria belong to the photosynthetic picoplankton and are probably the most abundant photosyn ...
'' and some ''Synechococcus
''Synechococcus'' (from the Greek ''synechos'', in succession, and the Greek ''kokkos'', granule) is a unicellular cyanobacterium that is very widespread in the marine environment. Its size varies from 0.8 to 1.5 μm. The photosynthetic ...
'' that can only take up nitrogen as ammonium.
Phosphorus is an essential nutrient for plants and animals. Phosphorus is a limiting nutrient
A limiting factor is a variable of a system that causes a noticeable change in output or another measure of a type of system. The limiting factor is in a pyramid shape of organisms going up from the producers to consumers and so on. A factor not l ...
for aquatic organisms. Phosphorus forms parts of important life-sustaining molecules that are very common in the biosphere. Phosphorus does enter the atmosphere in very small amounts when the dust is dissolved in rainwater and seaspray but remains mostly on land and in rock and soil minerals. Eighty per cent of the mined phosphorus is used to make fertilizers. Phosphates from fertilizers, sewage and detergents can cause pollution in lakes and streams. Over-enrichment of phosphate in both fresh and inshore marine waters can lead to massive algae bloom
An algal bloom or algae bloom is a rapid increase or accumulation in the population of algae in fresh water or marine water systems. It is often recognized by the discoloration in the water from the algae's pigments. The term ''algae'' encompass ...
s which, when they die and decay leads to eutrophication
Eutrophication is a general term describing a process in which nutrients accumulate in a body of water, resulting in an increased growth of organisms that may deplete the oxygen in the water; ie. the process of too many plants growing on the s ...
of freshwaters only. Recent research suggests that the predominant pollutant responsible for algal blooms in saltwater estuaries and coastal marine habitats is nitrogen.
Phosphorus occurs most abundantly in nature as part of the orthophosphate
In chemistry, a phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners ...
ion (PO4)3−, consisting of a P atom and 4 oxygen atoms. On land most phosphorus is found in rocks and minerals. Phosphorus-rich deposits have generally formed in the ocean or from guano, and over time, geologic processes bring ocean sediments to land. Weathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no move ...
of rocks and minerals release phosphorus in a soluble form where it is taken up by plants, and it is transformed into organic compounds. The plants may then be consumed by herbivore
A herbivore is an animal anatomically and physiologically evolved to feed on plants, especially upon vascular tissues such as foliage, fruits or seeds, as the main component of its diet. These more broadly also encompass animals that eat ...
s and the phosphorus is either incorporated into their tissues or excreted. After death, the animal or plant decays, and phosphorus is returned to the soil where a large part of the phosphorus is transformed into insoluble compounds. Runoff may carry a small part of the phosphorus back to the ocean
The ocean is the body of salt water that covers approximately 70.8% of Earth. The ocean is conventionally divided into large bodies of water, which are also referred to as ''oceans'' (the Pacific, Atlantic, Indian Ocean, Indian, Southern Ocean ...
.
Nutrient cycle
 A
A nutrient cycle
A nutrient cycle (or ecological recycling) is the movement and exchange of inorganic and organic matter back into the production of matter. Energy flow is a unidirectional and noncyclic pathway, whereas the movement of mineral nutrients is cyc ...
is the movement and exchange of organic and inorganic
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''.
Inor ...
matter back into the production
Production may refer to:
Economics and business
* Production (economics)
* Production, the act of manufacturing goods
* Production, in the outline of industrial organization, the act of making products (goods and services)
* Production as a stat ...
of matter. The process is regulated by the pathways available in marine food webs, which ultimately decompose organic matter back into inorganic nutrients. Nutrient cycles occur within ecosystems. Energy flow always follows a unidirectional and noncyclic path, whereas the movement of mineral nutrients
In the context of nutrition, a mineral is a chemical element. Some "minerals" are essential for life, but most are not. ''Minerals'' are one of the four groups of essential nutrients; the others are vitamins, essential fatty acids, and essenti ...
is cyclic. Mineral cycles include the carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
, oxygen cycle
The oxygen cycle refers to the various movements of oxygen through the Earth's atmosphere (air), biosphere (flora and fauna), hydrosphere (water bodies and glaciers) and the lithosphere (the Earth's crust). The oxygen cycle demonstrates how free ...
, nitrogen cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, atmospheric, terrestrial ecosystem, terrestrial, and marine ecosystems. The conversion of nitrogen can ...
, phosphorus cycle
The phosphorus cycle is the biogeochemical cycle that involves the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the moveme ...
and sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a consti ...
among others that continually recycle along with other mineral nutrients into productive
Productivity is the efficiency of production of goods or services expressed by some measure. Measurements of productivity are often expressed as a ratio of an aggregate output to a single input or an aggregate input used in a production proce ...
ecological nutrition.
There is considerable overlap between the terms for the biogeochemical cycle
A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cyc ...
and nutrient cycle. Some textbooks integrate the two and seem to treat them as synonymous terms. However, the terms often appear independently. Nutrient cycle is more often used in direct reference to the idea of an intra-system cycle, where an ecosystem functions as a unit. From a practical point, it does not make sense to assess a terrestrial ecosystem by considering the full column of air above it as well as the great depths of Earth below it. While an ecosystem often has no clear boundary, as a working model it is practical to consider the functional community where the bulk of matter and energy transfer occurs. Nutrient cycling occurs in ecosystems that participate in the "larger biogeochemical cycles of the earth through a system of inputs and outputs."
Dissolved nutrients
Nutrients dissolved in seawater are essential for the survival of marine life. Nitrogen and phosphorus are particularly important. They are regarded aslimiting nutrient
A limiting factor is a variable of a system that causes a noticeable change in output or another measure of a type of system. The limiting factor is in a pyramid shape of organisms going up from the producers to consumers and so on. A factor not l ...
s in many marine environments, because primary producers, like algae and marine plants, cannot grow without them. They are critical for stimulating primary production
In ecology, primary production is the synthesis of organic compounds from atmospheric or aqueous carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through ...
by phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
. Other important nutrients are silicon, iron, and zinc.Dissolved Nutrients''Earth in the Future'', PenState/NASSA. Retrieved 18 June 2020. The process of cycling nutrients in the sea starts with biological pumping, when nutrients are extracted from surface waters by phytoplankton to become part of their organic makeup. Phytoplankton are either eaten by other organisms, or eventually die and drift down as
marine snow
In the deep ocean, marine snow (also known as "ocean dandruff") is a continuous shower of mostly organic detritus falling from the upper layers of the water column. It is a significant means of exporting energy from the light-rich photic zone to ...
. There they decay and return to the dissolved state, but at greater ocean depths. The fertility of the oceans depends on the abundance of the nutrients, and is measured by the primary production
In ecology, primary production is the synthesis of organic compounds from atmospheric or aqueous carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through ...
, which is the rate of fixation of carbon per unit of water per unit time. "Primary production is often mapped by satellites using the distribution of chlorophyll, which is a pigment produced by plants that absorbs energy during photosynthesis. The distribution of chlorophyll is shown in the figure above. You can see the highest abundance close to the coastlines where nutrients from the land are fed in by rivers. The other location where chlorophyll levels are high is in upwelling zones where nutrients are brought to the surface ocean from depth by the upwelling process..."
"Another critical element for the health of the oceans is the dissolved oxygen content. Oxygen in the surface ocean is continuously added across the air-sea interface as well as by photosynthesis; it is used up in respiration by marine organisms and during the decay or oxidation of organic material that rains down in the ocean and is deposited on the ocean bottom. Most organisms require oxygen, thus its depletion has adverse effects for marine populations. Temperature also affects oxygen levels as warm waters can hold less dissolved oxygen than cold waters. This relationship will have major implications for future oceans, as we will see... The final seawater property we will consider is the content of dissolved . is nearly opposite to oxygen in many chemical and biological processes; it is used up by plankton during photosynthesis and replenished during respiration as well as during the oxidation of organic matter. As we will see later, content has importance for the study of deep-water aging."

Marine sulfur cycle
 Sulfate reduction in the seabed is strongly focused toward near-surface sediments with high depositional rates along the ocean margins. The benthic marine sulfur cycle is therefore sensitive to anthropogenic influence, such as ocean warming and increased nutrient loading of coastal seas. This stimulates photosynthetic productivity and results in enhanced export of organic matter to the seafloor, often combined with low oxygen concentration in the bottom water (Rabalais et al., 2014; Breitburg et al., 2018). The biogeochemical zonation is thereby compressed toward the sediment surface, and the balance of organic matter mineralization is shifted from oxic and suboxic processes toward sulfate reduction and methanogenesis (Middelburg and Levin, 2009).
*
Sulfate reduction in the seabed is strongly focused toward near-surface sediments with high depositional rates along the ocean margins. The benthic marine sulfur cycle is therefore sensitive to anthropogenic influence, such as ocean warming and increased nutrient loading of coastal seas. This stimulates photosynthetic productivity and results in enhanced export of organic matter to the seafloor, often combined with low oxygen concentration in the bottom water (Rabalais et al., 2014; Breitburg et al., 2018). The biogeochemical zonation is thereby compressed toward the sediment surface, and the balance of organic matter mineralization is shifted from oxic and suboxic processes toward sulfate reduction and methanogenesis (Middelburg and Levin, 2009).
* cable bacteria
Cable bacteria are filamentous bacteria that conduct electricity across distances over 1 cm in sediment and groundwater aquifers. Cable bacteria allow for long-distance electron transport, which connects electron donors to electron acceptors ...
The sulfur cycle in marine environments has been well-studied via the tool of sulfur isotope systematics expressed as δ34S. The modern global oceans have sulfur storage of 1.3 × 1021 g, mainly occurring as sulfate with the δ34S value of +21‰. The overall input flux is 1.0 × 1014 g/year with the sulfur isotope composition of ~3‰. Riverine sulfate derived from the terrestrial weathering of sulfide minerals (δ34S = +6‰) is the primary input of sulfur to the oceans. Other sources are metamorphic and volcanic degassing and hydrothermal activity (δ34S = 0‰), which release reduced sulfur species (e.g., H2S and S0). There are two major outputs of sulfur from the oceans. The first sink is the burial of sulfate either as marine evaporites (e.g., gypsum) or carbonate-associated sulfate (CAS), which accounts for 6 × 1013 g/year (δ34S = +21‰). The second sulfur sink is pyrite burial in shelf sediments or deep seafloor sediments (4 × 1013 g/year; δ34S = -20‰). The total marine sulfur output flux is 1.0 × 1014 g/year which matches the input fluxes, implying the modern marine sulfur budget is at steady state. The residence time of sulfur in modern global oceans is 13,000,000 years.
In modern oceans, '' Hydrogenovibrio crunogenus'', '' Halothiobacillus'', and ''Beggiatoa
''Beggiatoa'' is a genus of ''Gammaproteobacteria'' belonging to the order '' Thiotrichales'', in the ''Pseudomonadota'' phylum. These bacteria form colorless filaments composed of cells that can be up to 200 μm in diameter, and are one of ...
'' are primary sulfur oxidizing bacteria, and form chemosynthetic symbioses with animal hosts. The host provides metabolic substrates (e.g., CO2, O2, H2O) to the symbiont while the symbiont generates organic carbon for sustaining the metabolic activities of the host. The produced sulfate usually combines with the leached calcium ions to form gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk ...
, which can form widespread deposits on near mid-ocean spreading centers.
Hydrothermal vent
Hydrothermal vents are fissures on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hot ...
s emit hydrogen sulfide that support the carbon fixation of chemolithotrophic bacteria that oxidize hydrogen sulfide with oxygen to produce elemental sulfur or sulfate.
Iron cycle and dust
 The
The iron cycle
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient ...
(Fe) is the biogeochemical cycle of iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
through the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
, hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the Planetary surface, surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to ch ...
, biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
and lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the lithospheric mantle, the topmost portion of the upper mantle that behaves elastically on time ...
. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity
Primary or primaries may refer to:
Arts, entertainment, and media Music Groups and labels
* Primary (band), from Australia
* Primary (musician), hip hop musician and record producer from South Korea
* Primary Music, Israeli record label
Works
* ...
, and a limiting nutrient
A limiting factor is a variable of a system that causes a noticeable change in output or another measure of a type of system. The limiting factor is in a pyramid shape of organisms going up from the producers to consumers and so on. A factor not l ...
in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.
Iron in the ocean cycles between plankton, aggregated particulates (non-bioavailable iron), and dissolved (bioavailable iron), and becomes sediments through burial. Hydrothermal vent
Hydrothermal vents are fissures on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hot ...
s release ferrous iron to the ocean in addition to oceanic iron inputs from land sources. Iron reaches the atmosphere through volcanism, aeolian wind, and some via combustion by humans. In the Anthropocene
''Anthropocene'' is a term that has been used to refer to the period of time during which human impact on the environment, humanity has become a planetary force of change. It appears in scientific and social discourse, especially with respect to ...
, iron is removed from mines in the crust and a portion re-deposited in waste repositories.
 Iron is an essential micronutrient for almost every life form. It is a key component of hemoglobin, important to nitrogen fixation as part of the
Iron is an essential micronutrient for almost every life form. It is a key component of hemoglobin, important to nitrogen fixation as part of the Nitrogenase
Nitrogenases are enzymes () that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria) and rhizobacteria. These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only fa ...
enzyme family, and as part of the iron-sulfur core of ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied t ...
it facilitates electron transport in chloroplasts, eukaryotic mitochondria, and bacteria. Due to the high reactivity of Fe2+ with oxygen and low solubility of Fe3+, iron is a limiting nutrient in most regions of the world.
Calcium and silica cycles
The calcium cycle is a transfer of calcium between dissolved andsolid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
phases. There is a continuous supply of calcium ions
Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction ...
into waterways from rocks
In geology, rock (or stone) is any naturally occurring solid mass or aggregate of minerals or mineraloid matter. It is categorized by the minerals included, its chemical composition, and the way in which it is formed. Rocks form the Earth's ...
, organism
An organism is any life, living thing that functions as an individual. Such a definition raises more problems than it solves, not least because the concept of an individual is also difficult. Many criteria, few of them widely accepted, have be ...
s, and soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
s. Calcium ions are consumed and removed from aqueous environments as they react to form insoluble structures such as calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
and calcium silicate, which can deposit to form sediments or the exoskeleton
An exoskeleton () . is a skeleton that is on the exterior of an animal in the form of hardened integument, which both supports the body's shape and protects the internal organs, in contrast to an internal endoskeleton (e.g. human skeleton, that ...
s of organisms. Calcium ions can also be utilized biologically
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, origin, evolution, and distribution of ...
, as calcium is essential to biological functions such as the production of bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, ...
s and teeth
A tooth (: teeth) is a hard, calcified structure found in the jaws (or mouths) of many vertebrates and used to break down food. Some animals, particularly carnivores and omnivores, also use teeth to help with capturing or wounding prey, tear ...
or cellular function. The calcium cycle is a common thread between terrestrial, marine, geological, and biological processes. Calcium moves through these different media as it cycles throughout the Earth. The marine calcium cycle is affected by changing atmospheric carbon dioxide
In Earth's atmosphere, carbon dioxide is a trace gas that plays an integral part in the greenhouse effect, carbon cycle, photosynthesis and oceanic carbon cycle. It is one of three main greenhouse gases in the atmosphere of Earth. The concen ...
due to ocean acidification
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ...
.
Biogenic calcium carbonate is formed when marine organisms, such as coccolithophore
Coccolithophores, or coccolithophorids, are single-celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdom ...
s, coral
Corals are colonial marine invertebrates within the subphylum Anthozoa of the phylum Cnidaria. They typically form compact Colony (biology), colonies of many identical individual polyp (zoology), polyps. Coral species include the important Coral ...
s, pteropods, and other mollusks
Mollusca is a phylum of protostomic invertebrate animals, whose members are known as molluscs or mollusks (). Around 76,000 extant species of molluscs are recognized, making it the second-largest animal phylum after Arthropoda. The num ...
transform calcium ions and bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
into shells and exoskeleton
An exoskeleton () . is a skeleton that is on the exterior of an animal in the form of hardened integument, which both supports the body's shape and protects the internal organs, in contrast to an internal endoskeleton (e.g. human skeleton, that ...
s of calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
or aragonite
Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate (), the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation fr ...
, both forms of calcium carbonate. This is the dominant sink for dissolved calcium in the ocean. Dead organisms sink to the bottom of the ocean, depositing layers of shell which over time cement to form limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
. This is the origin of both marine and terrestrial limestone.
Calcium precipitates into calcium carbonate according to the following equation:
Ca2+ + 2HCO3− → CO2+ H2O + CaCO3
The relationship between dissolved calcium and calcium carbonate is affected greatly by the levels of carbon dioxide (CO2) in the atmosphere.
Increased carbon dioxide leads to more bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
in the ocean according to the following equation:
CO2 + CO32− + H2O → 2HCO3−

 With its close relation to the
With its close relation to the carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
and the effects of greenhouse gasses, both calcium and carbon cycles are predicted to change in the coming years. Tracking calcium isotopes enables the prediction of environmental changes, with many sources suggesting increasing temperatures in both the atmosphere and marine environment. As a result, this will drastically alter the breakdown of rock, the pH of oceans and waterways and thus calcium sedimentation, hosting an array of implications on the calcium cycle.
Due to the complex interactions of calcium with many facets of life, the effects of altered environmental conditions are unlikely to be known until they occur. Predictions can however be tentatively made, based upon evidence-based research. Increasing carbon dioxide levels and decreasing ocean pH will alter calcium solubility, preventing corals and shelled organisms from developing their calcium-based exoskeletons, thus making them vulnerable or unable to survive.
Most biological production of biogenic silica in the ocean is driven by diatom
A diatom (Neo-Latin ''diatoma'') is any member of a large group comprising several Genus, genera of algae, specifically microalgae, found in the oceans, waterways and soils of the world. Living diatoms make up a significant portion of Earth's B ...
s, with further contributions from radiolaria
The Radiolaria, also called Radiozoa, are unicellular eukaryotes of diameter 0.1–0.2 mm that produce intricate mineral skeletons, typically with a central capsule dividing the cell into the inner and outer portions of endoplasm and ect ...
ns. These microorganisms extract dissolved silicic acid
In chemistry, a silicic acid () is any chemical compound containing the element silicon attached to oxide () and hydroxyl () groups, with the general formula or, equivalently, . Orthosilicic acid is a representative example. Silicic acids are ra ...
from surface waters during growth, and return this by recycling throughout the water column
The (oceanic) water column is a concept used in oceanography to describe the physical (temperature, salinity, light penetration) and chemical ( pH, dissolved oxygen, nutrient salts) characteristics of seawater at different depths for a defined ...
after they die. Inputs of silicon to the ocean from above arrive via rivers and aeolian dust
Dust is made of fine particles of solid matter. On Earth, it generally consists of particles in the atmosphere that come from various sources such as soil lifted by wind (an aeolian process), volcanic eruptions, and pollution.
Dust in home ...
, while those from below include seafloor sediment recycling, weathering, and hydrothermal activity.
Biomineralization
 "Biological activity is a dominant force shaping the chemical structure and evolution of the earth surface environment. The presence of an oxygenated atmosphere-hydrosphere surrounding an otherwise highly reducing solid earth is the most striking consequence of the rise of life on earth. Biological evolution and the functioning of ecosystems, in turn, are to a large degree conditioned by geophysical and geological processes. Understanding the interactions between organisms and their abiotic environment, and the resulting coupled evolution of the biosphere and geosphere is a central theme of research in biogeology. Biogeochemists contribute to this understanding by studying the transformations and transport of chemical substrates and products of biological activity in the environment."
"Since the Cambrian explosion, mineralized body parts have been secreted in large quantities by biota. Because calcium carbonate, silica and calcium phosphate are the main mineral phases constituting these hard parts, biomineralization plays an important role in the global biogeochemical cycles of carbon, calcium, silicon and phosphorus"
"Biological activity is a dominant force shaping the chemical structure and evolution of the earth surface environment. The presence of an oxygenated atmosphere-hydrosphere surrounding an otherwise highly reducing solid earth is the most striking consequence of the rise of life on earth. Biological evolution and the functioning of ecosystems, in turn, are to a large degree conditioned by geophysical and geological processes. Understanding the interactions between organisms and their abiotic environment, and the resulting coupled evolution of the biosphere and geosphere is a central theme of research in biogeology. Biogeochemists contribute to this understanding by studying the transformations and transport of chemical substrates and products of biological activity in the environment."
"Since the Cambrian explosion, mineralized body parts have been secreted in large quantities by biota. Because calcium carbonate, silica and calcium phosphate are the main mineral phases constituting these hard parts, biomineralization plays an important role in the global biogeochemical cycles of carbon, calcium, silicon and phosphorus"
Deep cycling
 Deep cycling involves the exchange of materials with the mantle. The deep water cycle involves exchange of water with the mantle, with water carried down by
Deep cycling involves the exchange of materials with the mantle. The deep water cycle involves exchange of water with the mantle, with water carried down by subducting
Subduction is a geological process in which the oceanic lithosphere and some continental lithosphere is recycled into the Earth's mantle at the convergent boundaries between tectonic plates. Where one tectonic plate converges with a second pla ...
oceanic plates and returning through volcanic activity, distinct from the water cycle
The water cycle (or hydrologic cycle or hydrological cycle) is a biogeochemical cycle that involves the continuous movement of water on, above and below the surface of the Earth across different reservoirs. The mass of water on Earth remains fai ...
process that occurs above and on the surface of Earth. Some of the water makes it all the way to the lower mantle and may even reach the outer core
Earth's outer core is a fluid layer about thick, composed of mostly iron and nickel that lies above Earth's solid Earth's inner core, inner core and below its Earth's mantle, mantle. The outer core begins approximately beneath Earth's surface ...
.
In the conventional view of the water cycle (also known as the ''hydrologic cycle''), water moves between reservoirs in the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
and Earth's surface or near-surface (including the ocean
The ocean is the body of salt water that covers approximately 70.8% of Earth. The ocean is conventionally divided into large bodies of water, which are also referred to as ''oceans'' (the Pacific, Atlantic, Indian Ocean, Indian, Southern Ocean ...
, river
A river is a natural stream of fresh water that flows on land or inside Subterranean river, caves towards another body of water at a lower elevation, such as an ocean, lake, or another river. A river may run dry before reaching the end of ...
s and lake
A lake is often a naturally occurring, relatively large and fixed body of water on or near the Earth's surface. It is localized in a basin or interconnected basins surrounded by dry land. Lakes lie completely on land and are separate from ...
s, glacier
A glacier (; or ) is a persistent body of dense ice, a form of rock, that is constantly moving downhill under its own weight. A glacier forms where the accumulation of snow exceeds its ablation over many years, often centuries. It acquires ...
s and polar ice cap
A polar ice cap or polar cap is a high-latitude region of a planet, dwarf planet, or natural satellite that is covered in ice.
There are no requirements with respect to size or composition for a body of ice to be termed a polar ice cap, nor a ...
s, the biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
and groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit ...
). However, in addition to the surface cycle, water also plays an important role in geological processes reaching down into the crust and mantle. Water content in magma
Magma () is the molten or semi-molten natural material from which all igneous rocks are formed. Magma (sometimes colloquially but incorrectly referred to as ''lava'') is found beneath the surface of the Earth, and evidence of magmatism has also ...
determines how explosive a volcanic eruption is; hot water is the main conduit for economically important minerals to concentrate in hydrothermal mineral deposit Hydrothermal mineral deposits are accumulations of valuable minerals which formed from Hydrothermal circulation, hot waters circulating in Earth's Crust (geology), crust through fractures. They eventually produce metallic-rich fluids concentrated in ...
s; and water plays an important role in the formation and migration of petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
. Petroleum is a fossil fuel
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geolog ...
derived from ancient fossilized
A fossil (from Classical Latin , ) is any preserved remains, impression, or trace of any once-living thing from a past geological age. Examples include bones, shells, exoskeletons, stone imprints of animals or microbes, objects preserved ...
organic material
Organic matter, organic material or natural organic matter is the large source of carbon-based compounds found within natural and engineered, terrestrial, and aquatic environments. It is matter composed of organic compounds that have come fro ...
s, such as zooplankton
Zooplankton are the heterotrophic component of the planktonic community (the " zoo-" prefix comes from ), having to consume other organisms to thrive. Plankton are aquatic organisms that are unable to swim effectively against currents. Consequent ...
and algae
Algae ( , ; : alga ) is an informal term for any organisms of a large and diverse group of photosynthesis, photosynthetic organisms that are not plants, and includes species from multiple distinct clades. Such organisms range from unicellular ...
.
Water is not just present as a separate phase in the ground. Seawater percolates into oceanic crust and hydrates
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understo ...
igneous rocks such as olivine
The mineral olivine () is a magnesium iron Silicate minerals, silicate with the chemical formula . It is a type of Nesosilicates, nesosilicate or orthosilicate. The primary component of the Earth's upper mantle (Earth), upper mantle, it is a com ...
and pyroxene
The pyroxenes (commonly abbreviated Px) are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula , where X represents ions of calcium (Ca), sodium (Na), iron ( ...
, transforming them into hydrous minerals such as serpentines, talc
Talc, or talcum, is a clay mineral composed of hydrated magnesium silicate, with the chemical formula . Talc in powdered form, often combined with corn starch, is used as baby powder. This mineral is used as a thickening agent and lubricant ...
and brucite
Brucite is the mineral form of magnesium hydroxide, with the chemical formula Magnesium, Mg(hydroxyl, OH)2. It is a common alteration product of periclase in marble; a low-temperature hydrothermal Vein (geology), vein mineral in metamorphosed li ...
. In this form, water is carried down into the mantle. In the upper mantle
The upper mantle of Earth is a very thick layer of rock inside the planet, which begins just beneath the crust (geology), crust (at about under the oceans and about under the continents) and ends at the top of the lower mantle (Earth), lower man ...
, heat and pressure dehydrates these minerals, releasing much of it to the overlying mantle wedge
A mantle wedge is a triangular shaped piece of mantle that lies above a subducting tectonic plate and below the overriding plate. This piece of mantle can be identified using seismic velocity imaging as well as earthquake maps. Subducting oceanic ...
, triggering the melting of rock that rises to form volcanic arc
A volcanic arc (also known as a magmatic arc) is a belt of volcanoes formed above a subducting oceanic tectonic plate, with the belt arranged in an arc shape as seen from above. Volcanic arcs typically parallel an oceanic trench, with the arc ...
s. However, some of the "nominally anhydrous minerals" that are stable deeper in the mantle can store small concentrations of water in the form of hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
(OH−), and because they occupy large volumes of the Earth, they are capable of storing at least as much as the world's oceans.
The conventional view of the ocean's origin is that it was filled by outgassing from the mantle in the early Archean
The Archean ( , also spelled Archaean or Archæan), in older sources sometimes called the Archaeozoic, is the second of the four geologic eons of Earth's history of Earth, history, preceded by the Hadean Eon and followed by the Proterozoic and t ...
and the mantle has remained dehydrated ever since. However, subduction carries water down at a rate that would empty the ocean in 1–2 billion years. Despite this, changes in the global sea level over the past 3–4 billion years have only been a few hundred metres, much smaller than the average ocean depth of 4 kilometres. Thus, the fluxes of water into and out of the mantle are expected to be roughly balanced, and the water content of the mantle steady. Water carried into the mantle eventually returns to the surface in eruptions at mid-ocean ridge
A mid-ocean ridge (MOR) is a undersea mountain range, seafloor mountain system formed by plate tectonics. It typically has a depth of about and rises about above the deepest portion of an ocean basin. This feature is where seafloor spreading ...
s and hotspots
Hotspot, Hot Spot or Hot spot may refer to:
Places
* Hot Spot, Kentucky, a community in the United States
Arts, entertainment, and media Fictional entities
* Hot Spot (comics), a name for the DC Comics character Isaiah Crockett
* Hot Spot (Tr ...
. Estimates of the amount of water in the mantle range from to 4 times the water in the ocean.
The deep carbon cycle is the movement of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
through the Earth's mantle and core
Core or cores may refer to:
Science and technology
* Core (anatomy), everything except the appendages
* Core (laboratory), a highly specialized shared research resource
* Core (manufacturing), used in casting and molding
* Core (optical fiber ...
.
It forms part of the carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
and is intimately connected to the movement of carbon in the Earth's surface and atmosphere. By returning carbon to the deep Earth, it plays a critical role in maintaining the terrestrial conditions necessary for life to exist. Without it, carbon would accumulate in the atmosphere, reaching extremely high concentrations over long periods of time.
Rock cycle
Fossil fuels
Aquaticphytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater Aquatic ecosystem, ecosystems. The name comes from the Greek language, Greek words (), meaning 'plant', and (), mea ...
and zooplankton
Zooplankton are the heterotrophic component of the planktonic community (the " zoo-" prefix comes from ), having to consume other organisms to thrive. Plankton are aquatic organisms that are unable to swim effectively against currents. Consequent ...
that died and sedimented in large quantities under anoxic conditions millions of years ago began forming petroleum and natural gas as a result of anaerobic decomposition
Anaerobic digestion is a sequence of processes by which microorganisms break down biodegradable material in the absence of oxygen. The process is used for industrial or domestic purposes to manage waste or to produce fuels. Much of the fermen ...
(by contrast, terrestrial plant
A terrestrial plant is a plant that grows on, in or from land. Other types of plants are aquatic plant, aquatic (living in or on water), semiaquatic (living at edge or seasonally in water), epiphyte, epiphytic (living on other plants), and litho ...
s tended to form coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
and methane). Over geological time
The geologic time scale or geological time scale (GTS) is a representation of time based on the rock record of Earth. It is a system of chronological dating that uses chronostratigraphy (the process of relating strata to time) and geochronolo ...
this organic matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic pa ...
, mixed with mud
Mud (, or Middle Dutch) is loam, silt or clay mixed with water. Mud is usually formed after rainfall or near water sources. Ancient mud deposits hardened over geological time to form sedimentary rock such as shale or mudstone (generally cal ...
, became buried under further heavy layers of inorganic sediment. The resulting high temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
and pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
caused the organic matter to chemically alter
Alter may refer to:
Computing and technology
* ALTER, a command in older implementations of COBOL
* Alter (SQL), a command in a data definition language within SQL
Music
* ''Alter'' (album), 2002 album by Floater
* ''Alter'', a 2006 remix alb ...
, first into a waxy material known as kerogen
Kerogen is solid, insoluble organic matter in sedimentary rocks. It consists of a variety of organic materials, including dead plants, algae, and other microorganisms, that have been compressed and heated by geological processes. All the kero ...
, which is found in oil shale
Oil shale is an organic-rich Granularity, fine-grained sedimentary rock containing kerogen (a solid mixture of Organic compound, organic chemical compounds) from which liquid hydrocarbons can be produced. In addition to kerogen, general compos ...
s, and then with more heat into liquid and gaseous hydrocarbons in a process known as catagenesis.
Such organisms and their resulting fossil fuels typically have an age of millions of years, and sometimes more than 650 million years,Paul Mann, Lisa Gahagan, and Mark B. Gordon, "Tectonic setting of the world's giant oil and gas fields", in Michel T. Halbouty (ed.''Giant Oil and Gas Fields of the Decade, 1990–1999''
Tulsa, Okla.:
American Association of Petroleum Geologists
The American Association of Petroleum Geologists (AAPG) is one of the world's largest professional geological societies with about 17,000 members across 129 countries. The AAPG works to "advance the science of geology, especially as it relates to ...
, p. 50, accessed 22 June 2009. the energy released in combustion is still photosynthetic in origin.
Other cycles
Such as trace minerals, micronutrients, human-induced cycles for synthetic compounds such aspolychlorinated biphenyl
Polychlorinated biphenyls (PCBs) are organochlorine compounds with the formula Carbon, C12Hydrogen, H10−''x''Chloride, Cl''x''; they were once widely used in the manufacture of carbonless copy paper, as heat transfer fluids, and as dielectri ...
(PCB).
References
Sources
* *Further references
* {{biogeochemical cycle, state=expanded Biogeochemical cycle Geochemistry Biogeography Marine organisms Oceanography