MYBPC3 on:
[Wikipedia]
[Google]
[Amazon]
 ]
The myosin-binding protein C, cardiac-type is a
]
The myosin-binding protein C, cardiac-type is a
 ]
cMyBP-C regulates the positioning of myosin and actin for interaction and acts as a tether to the myosin S1 heads, limiting their mobility. This results in a decreased number of crossbridges formed, which hinders force generation, due to its N-terminal C1-M-C2 region interacting with the myosin-S2 domain. Furthermore, cMyBP-C contributes to the regulation of cardiac contraction at short sarcomere length and is required for complete relaxation in diastole.
Interactions of cMyBP-C with its binding partners vary with its
]
cMyBP-C regulates the positioning of myosin and actin for interaction and acts as a tether to the myosin S1 heads, limiting their mobility. This results in a decreased number of crossbridges formed, which hinders force generation, due to its N-terminal C1-M-C2 region interacting with the myosin-S2 domain. Furthermore, cMyBP-C contributes to the regulation of cardiac contraction at short sarcomere length and is required for complete relaxation in diastole.
Interactions of cMyBP-C with its binding partners vary with its
Mass spectrometry characterization of MYBPC3 at COPaKB
GeneReviews/NIH/NCBI/UW entry on Familial Hypertrophic Cardiomyopathy Overview
* {{PDB Gallery, geneid=4607 Human proteins
 ]
The myosin-binding protein C, cardiac-type is a
]
The myosin-binding protein C, cardiac-type is a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
that in humans is encoded by the ''MYBPC3'' gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
. This isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have uniqu ...
is expressed exclusively in heart muscle during human
Humans (''Homo sapiens'') or modern humans are the most common and widespread species of primate, and the last surviving species of the genus ''Homo''. They are Hominidae, great apes characterized by their Prehistory of nakedness and clothing ...
and mouse development, and is distinct from those expressed in slow skeletal muscle
Skeletal muscle (commonly referred to as muscle) is one of the three types of vertebrate muscle tissue, the others being cardiac muscle and smooth muscle. They are part of the somatic nervous system, voluntary muscular system and typically are a ...
( MYBPC1) and fast skeletal muscle ( MYBPC2).
Structure
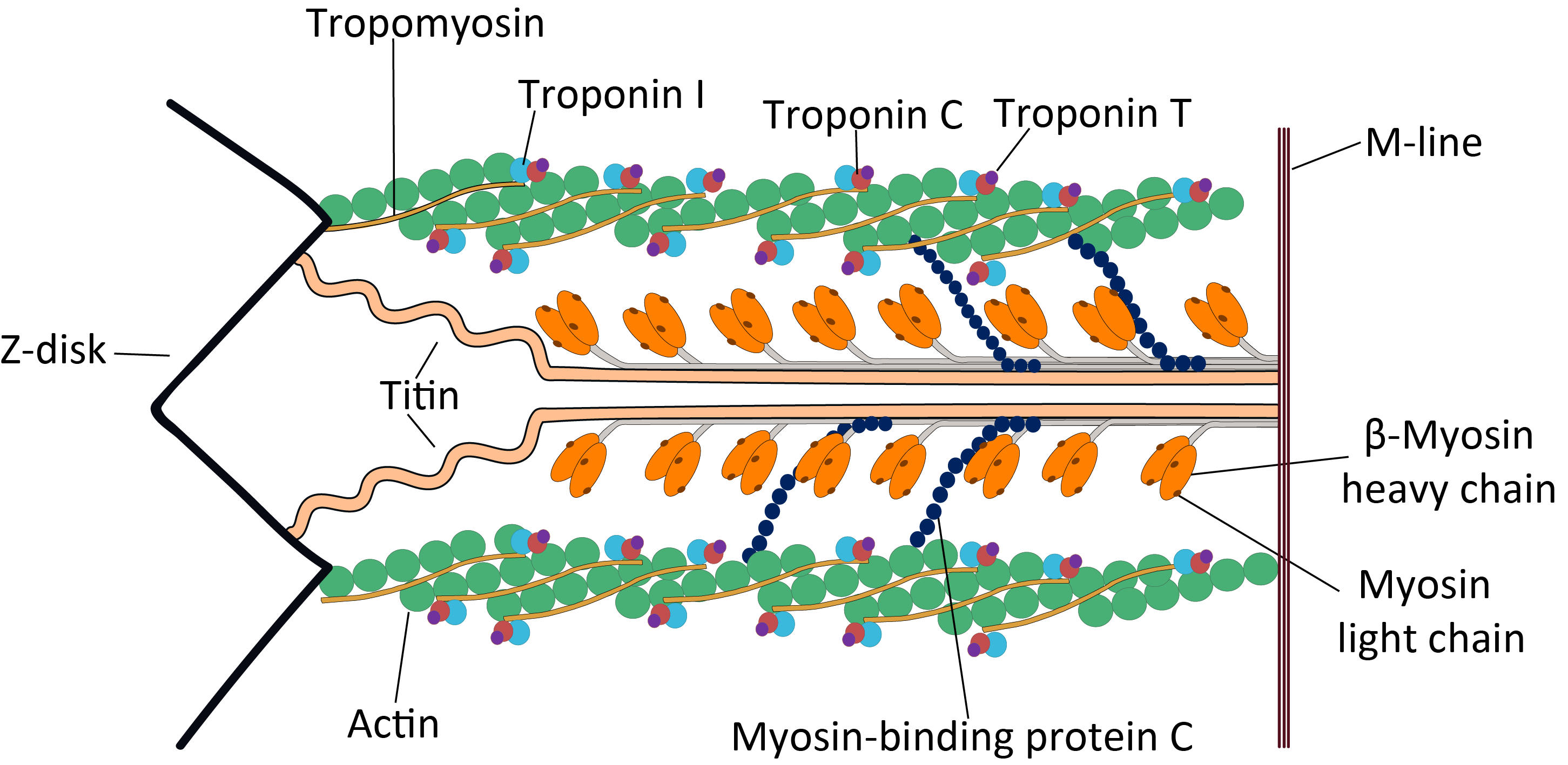
cMyBP-C is a 140.5 kDa protein composed of 1273 amino acids. cMyBP-C is a myosin-associated protein that binds at 43 nm intervals along the myosin thick filament backbone, stretching for 200 nm on either side of the M-line within the crossbridge-bearing zone (C-region) of theA band A band may refer to:
* A band (NATO)
The NATO A band is the obsolete designation given to the radio frequencies from 0 to 250 MHz (equivalent to wavelengths from 1.2 m upwards) during the cold war period. Since 1992, frequency allocations, ...
in striated muscle. The approximate stoichiometry of cMyBP-C along the thick filament is 1 per 9-10 myosin molecules, or 37 cMyBP-C molecules per thick filament. In addition to myosin, cMyBP-C also binds titin
Titin (; also called connectin) is a protein that in humans is encoded by the ''TTN'' gene. The protein, which is over 1 μm in length, functions as a molecular spring that is responsible for the passive elasticity of muscle. It comprises 2 ...
and actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of ...
. The cMyBP-C isoform expressed in cardiac muscle differs from those expressed in slow and fast skeletal muscle ( MYBPC1 and MYBPC2, respectively) by three features: (1) an additional immunoglobulin
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, includin ...
(Ig)-like domain on the N-terminus, (2) a linker region between the second and third Ig domains, and (3) an additional loop in the sixth Ig domain. cMyBP-C appears necessary for normal order, filament length and lattice spacing within the structure of the sarcomere
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal striated muscle, Skeletal muscles are composed of tubular ...
.
Function
cMyBP-C is not essential for sarcomere formation during embryogenesis, but is crucial for sarcomere organization and maintenance of normal cardiac function. Absence of cMyBP-C (''Mybpc3''-targeted knock-out mice) results in severe cardiac hypertrophy, increased heart-weight-to-body-weight-ratios, enlargement of ventricles, increased myofilament Ca2+ sensitivity and depressed diastolic and systolic function. Histologically, ''Mybpc3''-targeted knock-out hearts display structural rearrangements with cardiac myocyte disarray and increased interstitial fibrosis similar to patients withhypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM, or HOCM when obstructive) is a condition in which muscle tissues of the heart become thickened without an obvious cause. The parts of the heart most commonly affected are the interventricular septum and the ...
, without obvious alterations in shape or size of single cardiac myocytes. Ultrastructural examination revealed a loss of lateral alignment of adjacent myofibrils with their Z-lines misaligned.
cMyBP-C appears to act as a brake on cardiac contraction, as loaded shortening, power and cycling kinetics all increase in cMyBP-C knockout mice. Consistent with this notion, cMyBP-C knockout mice exhibit an abnormal systolic timecourse, with a shortened elastance timecourse and lower peak elastance in vivo, and an accelerated force development in isolated, skinned cardiac fibers suggesting that cMyBP-C is required to constrain the crossbridges in order to sustain a normal ejection.
 ]
cMyBP-C regulates the positioning of myosin and actin for interaction and acts as a tether to the myosin S1 heads, limiting their mobility. This results in a decreased number of crossbridges formed, which hinders force generation, due to its N-terminal C1-M-C2 region interacting with the myosin-S2 domain. Furthermore, cMyBP-C contributes to the regulation of cardiac contraction at short sarcomere length and is required for complete relaxation in diastole.
Interactions of cMyBP-C with its binding partners vary with its
]
cMyBP-C regulates the positioning of myosin and actin for interaction and acts as a tether to the myosin S1 heads, limiting their mobility. This results in a decreased number of crossbridges formed, which hinders force generation, due to its N-terminal C1-M-C2 region interacting with the myosin-S2 domain. Furthermore, cMyBP-C contributes to the regulation of cardiac contraction at short sarcomere length and is required for complete relaxation in diastole.
Interactions of cMyBP-C with its binding partners vary with its posttranslational modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translate mRNA ...
status. At least three extensively characterized phosphorylation
In biochemistry, phosphorylation is described as the "transfer of a phosphate group" from a donor to an acceptor. A common phosphorylating agent (phosphate donor) is ATP and a common family of acceptor are alcohols:
:
This equation can be writ ...
sites (Ser273, 282 and 302; numbering refers to the mouse sequence) are localized in the M motif of cMyBP-C and are targeted by protein kinases
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a fun ...
in a hierarchical order of events. In its dephosphorylated state, cMyBP-C binds predominantly to myosin S2 and brakes crossbridge formation, however, when phosphorylated in response to β-adrenergic stimulation through activating cAMP-dependent protein kinase (PKA
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:H ...
), it favours binding to actin, then accelerating crossbridge formation, enhancing force development and promoting relaxation. Protein kinases identified thus far to phosphorylate cMyBP-C in the M motif are PKA
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:H ...
, Ca2+/calmodulin-dependent kinase II (CaMKII
/calmodulin-dependent protein kinase II (CaM kinase II or CaMKII) is a serine/threonine-specific protein kinase that is regulated by the /calmodulin complex. CaMKII is involved in many signaling cascades and is thought to be an important mediat ...
), ribosomal s6 kinase
In molecular biology, ribosomal s6 kinase (rsk) is a family of protein kinases involved in signal transduction. There are two subfamilies of rsk, p90rsk, also known as MAPK-activated protein kinase-1 (MAPKAP-K1), and p70rsk, also known as S6-H1 ...
(RSK), protein kinase D (PKD), and protein kinase C
In cell biology, protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and t ...
(PKC). Furthermore, GSK3β
Glycogen synthase kinase-3 beta, (GSK-3 beta), is an enzyme that in humans is encoded by the ''GSK3B'' gene. In mice, the enzyme is encoded by the Gsk3b gene. Abnormal regulation and expression of GSK-3 beta is associated with an increased susce ...
was described as another protein kinase to phosphorylate cMyBP-C outside the M-domain in the proline-alanine-rich actin-binding site at Ser133 in human myocardium (mouse Ser131). Phosphorylation is required for normal cardiac function and cMyBP-C stability, and overall phosphorylation levels of cMyBP-C are reduced in human and experimental heart failure. Other posttranslational modifications of cMyBP-C exist, which occur throughout the protein and are not thoroughly characterised yet, such as acetylation, citrullination, S-glutathiolation, S-nitrosylation and carbonylation.
Genetics
The cloning of the human ''MYBPC3'' cDNA and localization of the gene on human chromosome 11p11.2 has assisted the structure and function of cMyBP-C. ''MYBPC3'' became therefore the “best” candidate gene for the ''CMH4'' locus forhypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM, or HOCM when obstructive) is a condition in which muscle tissues of the heart become thickened without an obvious cause. The parts of the heart most commonly affected are the interventricular septum and the ...
that was initially mapped by the group of Schwartz. ''MYBPC3'' mutations segregating in families with hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM, or HOCM when obstructive) is a condition in which muscle tissues of the heart become thickened without an obvious cause. The parts of the heart most commonly affected are the interventricular septum and the ...
have been identified. ''MYBPC3'' was thus the fourth gene for hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM, or HOCM when obstructive) is a condition in which muscle tissues of the heart become thickened without an obvious cause. The parts of the heart most commonly affected are the interventricular septum and the ...
, following MYH7
Myosin-7 is a protein that in humans is encoded by the ''MYH7'' gene.
It is the myosin heavy chain beta (MHC-β) isoform (slow twitch) expressed primarily in the heart, but also in skeletal muscles (type I fibers). This isoform is distinct from ...
, encoding β- myosin heavy chain, TNNT2 and TPM1, encoding cardiac troponin T
Troponin T (shortened TnT or TropT) is a part of the troponin complex, which are proteins integral to the contraction of skeletal and heart muscles. They are expressed in skeletal and cardiac myocytes. Troponin T binds to tropomyosin and help ...
and α-tropomyosin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in many animal and fungal cells. In animals, it is an important component of the muscular system which works in conjunction with troponin to regulate muscle contraction. It ...
, respectively, earmarking hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM, or HOCM when obstructive) is a condition in which muscle tissues of the heart become thickened without an obvious cause. The parts of the heart most commonly affected are the interventricular septum and the ...
(HCM) as a disease of the sarcomere
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal striated muscle, Skeletal muscles are composed of tubular ...
. Truncation mutations in MYBPC3 stand as the primary cause of HCM.
To date, roughly 350 mutations in ''MYBPC3'' have been identified, and in large part, the mutations result in protein truncation, shifts in reading frames, and premature termination codons. Genetic studies have revealed significant overlap between genotypes and phenotypes as ''MYBPC3'' mutations can lead to various forms of cardiomyopathies, such as dilated cardiomyopathy
Dilated cardiomyopathy (DCM) is a condition in which the heart becomes enlarged and cannot pump blood effectively. Symptoms vary from none to feeling tired, leg swelling, and shortness of breath. It may also result in chest pain or fainting. C ...
and left ventricular noncompaction cardiomyopathy. In patients with isolated or familial cases of dilated cardiomyoathy, ''MYBPC3'' mutations represented the second highest number of known mutations. Furthermore, a 25-bp intronic ''MYBPC3'' deletion leading to protein truncation is present in 4% of the population in South India and is associated with a higher risk to develop heart failure. Founder ''MYBPC3'' mutations have been reported in Iceland, Italy, The Netherlands, Japan, France and Finland, where they represent a large percentage of cases with hypertrophic cardiomyopathy. All of them are truncating mutations, resulting in a shorter protein, lacking the regulatory phosphorylatable M motif and/or major binding domains to other sarcomeric proteins. A body of evidence indicates that patients with more than 1 mutation often develop a more severe phenotype, and a significant fraction of childhood-onset hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM, or HOCM when obstructive) is a condition in which muscle tissues of the heart become thickened without an obvious cause. The parts of the heart most commonly affected are the interventricular septum and the ...
(14%) is caused by compound genetic variants. This suggests that a gene-dosage effect might be responsible for manifestations at a younger age. A total of 51 cases of homozygotes or compound heterozygotes have been reported, most of them with double truncating ''MYBPC3'' mutations and associated with severe cardiomyopathy, leading to heart failure and death within the first year of life.
Pathomechanisms
A great understanding of how ''MYBPC3'' mutations lead to the development of inherited cardiomyopathy came from the analyses of human myocardial samples, gene transfer in different cell lines, naturally-occurring or transgenic animal models and more recently disease modeling using induced pluripotent stem cells (iPSC)-derived cardiac myocytes. Although access to human myocardial samples is difficult, at least some studies provided evidence that truncated cMyBP-Cs, resulting from truncating ''MYBPC3'' mutations are not detectable in human patient samples by Western-immunoblot analysis. This was supported in heterozygous ''Mybpc3''-targeted knock-in mice, carrying the human c.772G>A transition (i.e. founder mutation in Tuscany These data suggesthaploinsufficiency
Haploinsufficiency in genetics describes a model of dominant gene action in diploid organisms, in which a single copy of the wild-type allele at a locus in heterozygous combination with a variant allele is insufficient to produce the wild-type ...
as the main disease mechanism for heterozygous truncating mutations. A body of evidence exists that the mechanisms regulating the expression of mutant allele involve the nonsense-mediated mRNA decay, the ubiquitin-proteasome system (UPS) and the autophagy-lysosomal pathway after gene transfer of mutant ''MYBPC3'' in cardiac myocytes or in mice ''in vivo''. In contrast to truncating mutations, missense mutations lead, in most of the cases (although difficult to specifically detect), to stable mutant cMyBP-Cs that are, at least in part, incorporated into the sarcomere and could act as poison polypeptides on the structure and/or function of the sarcomere
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal striated muscle, Skeletal muscles are composed of tubular ...
. Homozygous or compound heterozygous mutations are therefore likely subject to differential regulation depending on whether they are double missense, double truncating or mixed missense/truncating mutations. The homozygous ''Mybpc3''-targeted knock-in mice, which genetically mimic the situation of severe neonatal cardiomyopathy are born without phenotype and soon after birth develop systolic dysfunction followed by (compensatory) cardiac hypertrophy. The human c.772G>A transition results in low levels of three different mutant ''Mybpc3'' mRNAs and cMyBP-Cs in homozygous mice, suggesting a combination of haploinsufficiency
Haploinsufficiency in genetics describes a model of dominant gene action in diploid organisms, in which a single copy of the wild-type allele at a locus in heterozygous combination with a variant allele is insufficient to produce the wild-type ...
and polypeptide poisoning as disease mechanism in the homozygous state. In addition, the combination of external stress (such as neurohumoral stress or aging) and ''Mybpc3'' mutations have been shown to impair the UPS in mice, and proteasomal activities were also depressed in patients with hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM, or HOCM when obstructive) is a condition in which muscle tissues of the heart become thickened without an obvious cause. The parts of the heart most commonly affected are the interventricular septum and the ...
or dilated cardiomyopathy
Dilated cardiomyopathy (DCM) is a condition in which the heart becomes enlarged and cannot pump blood effectively. Symptoms vary from none to feeling tired, leg swelling, and shortness of breath. It may also result in chest pain or fainting. C ...
.
Skinned trabeculae or cardiac myocytes obtained from human patients carrying a ''MYBPC3'' mutation or from heterozygous and homozygous ''Mybpc3''-targeted knock-in mice exhibited higher myofilament Ca2+ sensitivity than controls. Disease-modeling by engineered heart tissue (EHT) technology with cardiac cells from heterozygous or homozygous ''Mybpc3''-targeted knock-in mice reproduced observations made in human and mouse studies displaying abbreviated contractions, greater sensitivity to external Ca2+ and smaller inotropic responses to various drugs (isoprenaline
Isoprenaline, also known as isoproterenol and sold under the brand name Isuprel among others, is a sympathomimetic medication which is used in the treatment of acute bradycardia (slow heart rate), heart block, and rarely for asthma, among other ...
, EMD 57033 and verapamil
Verapamil, sold under various trade names, is a calcium channel blocker medication used for the treatment of high blood pressure, angina (chest pain from not enough blood flow to the heart), and supraventricular tachycardia. It may also be use ...
) compared to wild-type control EHTs. Therefore, EHTs are suitable to model the disease phenotype and recapitulate functional alterations found in mice with hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM, or HOCM when obstructive) is a condition in which muscle tissues of the heart become thickened without an obvious cause. The parts of the heart most commonly affected are the interventricular septum and the ...
. Another good system for modeling cardiomyopathies in the cell culture dish is the derivation of cardiac myocytes from iPSC. Reports of human iPSC models of sarcomeric cardiomyopathies showed cellular hypertrophy in most of the cases, including one with the c.2995_3010del ''MYBPC3'' mutation that exhibited in addition to hypertrophy contractile variability in the presence of endothelin-1
Endothelin 1 (ET-1), also known as preproendothelin-1 (PPET1), is a potent vasoconstrictor peptide produced by vascular endothelial cells, as well as by cells in the heart (affecting contractility) and kidney (affecting sodium handling). The prote ...
.
Therapy
Because of their tissue selectivity and persistent expression recombinantadeno-associated virus
Adeno-associated viruses (AAV) are small viruses that infect humans and some other primate species. They belong to the genus '' Dependoparvovirus'', which in turn belongs to the family ''Parvoviridae''. They are small (approximately 26 nm in ...
es (AAV) have therapeutic potential in the treatment of inherited cardiomyopathy resulting from ''MYBPC3'' mutations- Several targeting approaches have been developed. The most recent is genome editing to correct a mutation by CRISPR/Cas9 technology. Naturally existing as part of the prokaryotic immune system, the CRISPR/Cas9 system has been used for correction of mutations in the mammalian genome. By inducing nicks in the double-stranded DNA and providing a template DNA sequence, it is possible to repair mutations by homologous recombination
Homologous recombination is a type of genetic recombination in which genetic information is exchanged between two similar or identical molecules of double-stranded or single-stranded nucleic acids (usually DNA as in Cell (biology), cellular organi ...
. This approach has not yet been evaluated for ''MYBPC3'' mutations, but it could be used for each single or clustered mutation, and therefore applied preferentially for frequent founder ''MYBPC3'' mutations.
Other strategies targeting the mutant pre-mRNA by exon skipping
In molecular biology, exon skipping is a form of RNA splicing used to cause cells to “skip” over faulty or misaligned sections (exons) of genetic code, leading to a truncated but still functional protein despite the genetic mutation.
Mechanis ...
and/or spliceosome
A spliceosome is a large ribonucleoprotein (RNP) complex found primarily within the nucleus of eukaryotic cells. The spliceosome is assembled from small nuclear RNAs ( snRNA) and numerous proteins. Small nuclear RNA (snRNA) molecules bind to sp ...
-mediated RNA trans-splicing
''Trans''-splicing is a special form of RNA processing where exons from two different primary RNA transcripts are joined end to end and ligated. It is usually found in eukaryotes and mediated by the spliceosome, although some bacteria and archa ...
(SMaRT) have been evaluated for ''MYBPC3''. Exon skipping
In molecular biology, exon skipping is a form of RNA splicing used to cause cells to “skip” over faulty or misaligned sections (exons) of genetic code, leading to a truncated but still functional protein despite the genetic mutation.
Mechanis ...
can be achieved using antisense oligonucleotide (AON) masking exonic splicing enhancer sequences and therefore preventing binding of the splicing machinery and therefore resulting in exclusion of the exon from the mRNA. This approach can be applied when the resulting shorter, but in-frame translated protein maintains its function. Proof-of-concept of exon skipping
In molecular biology, exon skipping is a form of RNA splicing used to cause cells to “skip” over faulty or misaligned sections (exons) of genetic code, leading to a truncated but still functional protein despite the genetic mutation.
Mechanis ...
was recently shown in homozygous ''Mybpc3''-targeted knock-in mice. Systemic administration of AAV-based AONs to ''Mybpc3''-targeted knock-in newborn mice prevented both systolic dysfunction and left ventricular hypertrophy, at least for the duration of the investigated period. For the human ''MYBPC3'' gene, skipping of 6 single exons or 5 double exons with specific AONs would result in shortened in-frame cMyBP-Cs, allowing the preservation of the functionally important phosphorylation and protein interaction sites. With this approach, about half of missense or exonic/intronic truncating mutations could be removed, including 35 mutations in exon 25. The other strategy targeting the mutant pre-mRNA is SMaRT. Hereby, two independently transcribed molecules, the mutant pre-mRNA and the therapeutic pre-trans-splicing molecule carrying the wild-type sequence are spliced together to give rise to a repaired full-length mRNA. Recently, the feasibility of this method was shown both in isolated cardiac myocytes and ''in vivo'' in the heart of homozygous ''Mybpc3''-targeted knock-in mice, although the efficiency of the process was low and the amount of repaired protein was not sufficient to prevent the development of the cardiac disease phenotype. In principle, however, this SmART strategy is superior to exon skipping
In molecular biology, exon skipping is a form of RNA splicing used to cause cells to “skip” over faulty or misaligned sections (exons) of genetic code, leading to a truncated but still functional protein despite the genetic mutation.
Mechanis ...
or CRISPR/Cas9 genome editing and still attractive, because only two pre-trans-splicing molecules, targeting the 5’ and the 3’ of ''MYBPC3'' pre-mRNA would be sufficient to bypass all ''MYBPC3'' mutations associated with cardiomyopathies and therefore repair the mRNA.
AAV-mediated gene transfer of the full-length ''Mybpc3'' (defined as “gene replacement”) dose-dependently prevents the development of cardiac hypertrophy and dysfunction in homozygous ''Mybpc3''-targeted knock-in mice. The dose-dependent expression of exogenous ''Mybpc3'' was associated with the down-regulation of endogenous mutant ''Mybpc3''. Additional expression of a sarcomeric protein is expected to replace partially or completely the endogenous protein level in the sarcomere, as it has been shown in transgenic mice expressing sarcomeric proteins.
Notes
References
Further reading
* * * * * * * * * * * * * * * * *External links
Mass spectrometry characterization of MYBPC3 at COPaKB
GeneReviews/NIH/NCBI/UW entry on Familial Hypertrophic Cardiomyopathy Overview
* {{PDB Gallery, geneid=4607 Human proteins