List Of Interstellar And Circumstellar Molecules on:
[Wikipedia]
[Google]
[Amazon]
 This is a list of
This is a list of
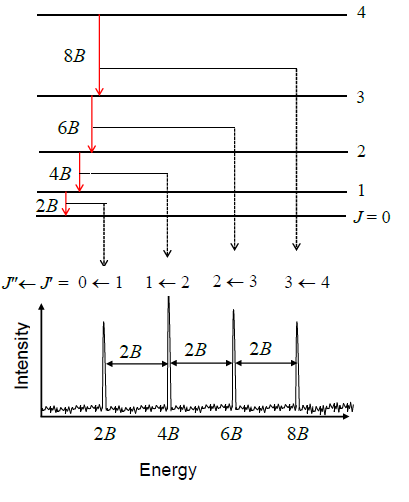 The molecules listed below were detected through
The molecules listed below were detected through  One of the richest sources for detecting interstellar molecules is Sagittarius B2 (Sgr B2), a
One of the richest sources for detecting interstellar molecules is Sagittarius B2 (Sgr B2), a





- This spectral assignment has not been independently confirmed, and is described by the authors as "tentative" (page L58).
 This is a list of
This is a list of molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s that have been detected in the interstellar medium
The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It f ...
and circumstellar envelope
A circumstellar envelope (CSE) is a part of a star that has a roughly spherical shape and is not gravitationally bound to the star core. Usually circumstellar envelopes are formed from the dense stellar wind, or they are present before the formati ...
s, grouped by the number of component atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s. The chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
is listed for each detected compound, along with any ionized form that has also been observed.
Background
 The molecules listed below were detected through
The molecules listed below were detected through astronomical spectroscopy
Astronomical spectroscopy is the study of astronomy using the techniques of spectroscopy to measure the electromagnetic spectrum, spectrum of electromagnetic radiation, including Visible light astronomy, visible light, Ultraviolet astronomy, ultr ...
. Their spectral features arise because molecules either absorb or emit a photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
of light when they transition between two molecular energy levels
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
. The energy (and thus the wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
) of the photon matches the energy difference between the levels involved. Molecular electronic transitions occur when one of the molecule's electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s moves between molecular orbitals
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
, producing a spectral line
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission (electromagnetic radiation), emission or absorption (electromagnetic radiation), absorption of light in a narrow frequency ...
in the ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of ...
, optical
Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behaviour of visible, ultravio ...
or near-infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those of ...
parts of the electromagnetic spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high ...
. Alternatively, a vibrational transition transfers quanta of energy to (or from) vibrations of molecular bonds, producing signatures in the mid- or far-infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
. Gas-phase molecules also have quantised rotational levels, leading to transitions at microwave
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequency, frequencies between 300&n ...
or radio
Radio is the technology of communicating using radio waves. Radio waves are electromagnetic waves of frequency between 3 hertz (Hz) and 300 gigahertz (GHz). They are generated by an electronic device called a transmitter connec ...
wavelengths.
Sometimes a transition can involve more than one of these types of energy level e.g. ro-vibrational spectroscopy changes both the rotational and vibrational energy level. Occasionally all three occur together, as in the Phillips band of C2 (diatomic carbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed throug ...
), in which an electronic transition produces a line in the near-infrared, which is then split into several vibronic bands by a simultaneous change in vibrational level, which in turn are split again into rotational branches.
The spectrum of a particular molecule is governed by the selection rules
In physics and chemistry, a selection rule, or transition rule, formally constrains the possible transitions of a system from one quantum state to another. Selection rules have been derived for electromagnetic transitions in molecules, in atoms, in ...
of quantum chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
and by its molecular symmetry
In chemistry, molecular symmetry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explai ...
. Some molecules have simple spectra which are easy to identify, whilst others (even some small molecules) have extremely complex spectra with flux spread among many different lines, making them far harder to detect. Interactions between the atomic nuclei and the electrons sometimes cause further hyperfine structure
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate electronic energy levels and the resulting splittings in those electronic energy levels of atoms, molecules, and ions, due to electromagnetic multipole int ...
of the spectral lines. If the molecule exists in multiple isotopologues (versions containing different atomic isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s), the spectrum is further complicated by isotope shifts.
Detection of a new interstellar or circumstellar molecule requires identifying a suitable astronomical object where it is likely to be present, then observing it with a telescope
A telescope is a device used to observe distant objects by their emission, Absorption (electromagnetic radiation), absorption, or Reflection (physics), reflection of electromagnetic radiation. Originally, it was an optical instrument using len ...
equipped with a spectrograph
An optical spectrometer (spectrophotometer, spectrograph or spectroscope) is an instrument used to measure properties of light over a specific portion of the electromagnetic spectrum, typically used in spectroscopic analysis to identify mate ...
working at the required wavelength, spectral resolution
The spectral resolution of a spectrograph, or, more generally, of a frequency spectrum, is a measure of its ability to resolve features in the electromagnetic spectrum. It is usually denoted by \Delta\lambda, and is closely related to the resolvi ...
and sensitivity. The first molecule detected in the interstellar medium was the methylidyne radical
Methylidyne, or (unsubstituted) carbyne, is an organic compound whose molecule consists of a single hydrogen atom bonded to a carbon atom. It is the parent compound of the carbynes, which can be seen as obtained from it by substitution of other ...
(CH•) in 1937, through its strong electronic transition at 4300 angstroms (in the optical). Advances in astronomical instrumentation have led to increasing numbers of new detections. From the 1950s onwards, radio astronomy
Radio astronomy is a subfield of astronomy that studies Astronomical object, celestial objects using radio waves. It started in 1933, when Karl Jansky at Bell Telephone Laboratories reported radiation coming from the Milky Way. Subsequent observat ...
began to dominate new detections, with sub-mm astronomy also becoming important from the 1990s.
The inventory of detected molecules is highly biased towards certain types which are easier to detect. For example, radio astronomy is most sensitive to small linear molecules with a high molecular dipole. The most common molecule in the Universe, H2 (molecular hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
), is completely invisible to radio telescopes because it has no dipole; its electronic transitions are too energetic for optical telescopes, so detection of H2 required ultraviolet observations with a sounding rocket
A sounding rocket or rocketsonde, sometimes called a research rocket or a suborbital rocket, is an instrument-carrying rocket designed to take measurements and perform scientific experiments during its sub-orbital flight. The rockets are often ...
. Vibrational lines are often not specific to an individual molecule, allowing only the general class to be identified. For example, the vibrational lines of polycyclic aromatic hydrocarbon
A Polycyclic aromatic hydrocarbon (PAH) is any member of a class of organic compounds that is composed of multiple fused aromatic rings. Most are produced by the incomplete combustion of organic matter— by engine exhaust fumes, tobacco, incine ...
s (PAHs) were identified in 1984, showing the class of molecules is very common in space, but it took until 2021 to identify any specific PAHs through their rotational lines.
 One of the richest sources for detecting interstellar molecules is Sagittarius B2 (Sgr B2), a
One of the richest sources for detecting interstellar molecules is Sagittarius B2 (Sgr B2), a giant molecular cloud
A molecular cloud—sometimes called a stellar nursery if star formation is occurring within—is a type of interstellar cloud of which the density and size permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen ...
near the centre of the Milky Way
The Milky Way or Milky Way Galaxy is the galaxy that includes the Solar System, with the name describing the #Appearance, galaxy's appearance from Earth: a hazy band of light seen in the night sky formed from stars in other arms of the galax ...
. About half of the molecules listed below were first found in Sgr B2, and many of the others have been subsequently detected there. Many of the largest molecules were first detected in another molecular cloud, TMC-1. A rich source of circumstellar molecules is CW Leonis (also known as IRC +10216), a nearby carbon star
A carbon star (C-type star) is typically an asymptotic giant branch star, a luminous red giant, whose Stellar atmosphere, atmosphere contains more carbon than oxygen. The two elements combine in the upper layers of the star, forming carbon monox ...
, where about 50 molecules have been identified. There is no clear boundary between interstellar and circumstellar media, so both are included in the tables below.
The discipline of astrochemistry
Astrochemistry is the study of the abundance and reactions of molecules in the universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar Syst ...
includes understanding how these molecules form and explaining their abundances. The extremely low density of the interstellar medium
The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It f ...
is not conducive to the formation of molecules, making conventional gas-phase reactions between neutral species (atoms or molecules) inefficient. Many regions also have very low temperatures (typically 10 kelvin
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By de ...
inside a molecular cloud), further reducing the reaction rates, or high ultraviolet radiation fields, which destroy molecules through photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 Nanometre, nm), visible ligh ...
. Explaining the observed abundances of interstellar molecules requires calculating the balance between formation and destruction rates using gas-phase ion chemistry (often driven by cosmic ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the ...
s), surface chemistry
Surface science is the study of physics, physical and chemistry, chemical phenomena that occur at the interface (chemistry), interface of two phase (matter), phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum int ...
on cosmic dust
Cosmic dustalso called extraterrestrial dust, space dust, or star dustis dust that occurs in outer space or has fallen onto Earth. Most cosmic dust particles measure between a few molecules and , such as micrometeoroids (30 μm). Cosmic dust can ...
, radiative transfer including interstellar extinction, and sophisticated reaction networks. The use of molecular lines to determine the physical properties of astronomical objects is known as molecular astrophysics.
Molecules
The following tables list molecules that have been detected in the interstellar medium or circumstellar matter, grouped by the number of componentatom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s. Neutral molecules and their molecular ions are listed in separate columns; if there is no entry in the molecule column, only the ionized form has been detected. Designations (names of molecules) are those used in the scientific literature describing the detection; if none was given that field is left empty. Mass is listed in atomic mass unit
The dalton or unified atomic mass unit (symbols: Da or u, respectively) is a unit of mass defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. It is a non-SI unit accepted ...
s. Deuterated molecules, which contain at least one deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more c ...
(2H) atom, have slightly different masses and are listed in a separate table. The total number of unique species, including distinct ionization states, is indicated in each section header.
Most of the molecules detected so far are organic. The only detected inorganic molecule with five or more atoms is SiH4. Molecules larger than that all have at least one carbon atom, with no N−N or O−O bonds.
Diatomic (45)

Triatomic (45)

Four atoms (31)

Five atoms (21)
Six atoms (16)

Seven atoms (16)

Eight atoms (14)

Nine atoms (11)
Ten or more atoms (24)
Deuterated molecules (22)
These molecules all contain one or moredeuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more c ...
atoms, a heavier isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
of hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
.
Unconfirmed (16)
Evidence for the existence of the following molecules has been reported in the scientific literature, but the detections either are described as tentative by the authors, or have been challenged by other researchers. They await independent confirmation.See also
*Astrochemistry
Astrochemistry is the study of the abundance and reactions of molecules in the universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar Syst ...
* Cosmic dust
Cosmic dustalso called extraterrestrial dust, space dust, or star dustis dust that occurs in outer space or has fallen onto Earth. Most cosmic dust particles measure between a few molecules and , such as micrometeoroids (30 μm). Cosmic dust can ...
* Diffuse interstellar band
* Lists of molecules
* Molecular astrophysics
* Molecular spectroscopy
* Molecules in stars
* Polycyclic aromatic hydrocarbon (PAH)
* Tholin
Notes
References
External links
* * * * * * {{DEFAULTSORT:List of interstellar and circumstellar molecules Astrochemistry Molecules in interstellar space Interstellar media Molecules