Iron Ore on:
[Wikipedia]
[Google]
[Amazon]

 Iron ores are rocks and
Iron ores are rocks and
 Iron ore represents 93% of metals mined worldwide in 2021. Steel, of which iron is the key ingredient, represents almost 95% of all metal used per year.Iron ore pricing emerges from stone age
Iron ore represents 93% of metals mined worldwide in 2021. Steel, of which iron is the key ingredient, represents almost 95% of all metal used per year.Iron ore pricing emerges from stone age
''Financial Times'', October 26, 2009 Iron-rich rocks are common worldwide, but ore-grade commercial
''Financial Times'', October 14, 2009 It is highly capital intensive, and requires significant investment in infrastructure such as rail in order to transport the ore from the mine to a freight ship. For these reasons, iron ore production is concentrated in the hands of a few major players. World production averages of raw ore annually. The world's largest producer of iron ore is the Brazilian mining corporation
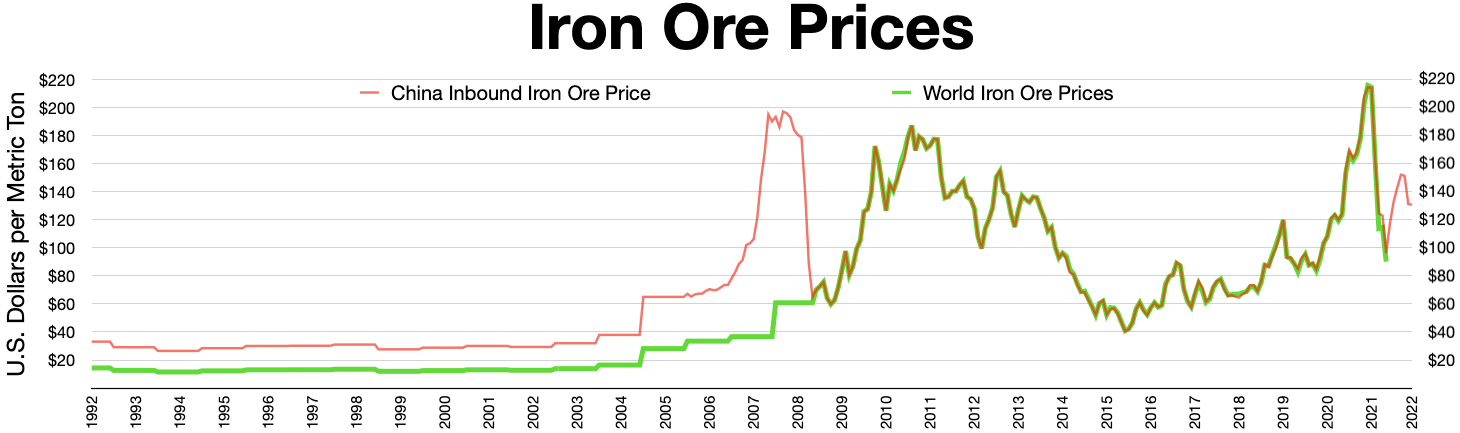
 Over the last 40 years, iron ore prices have been decided in closed-door negotiations between the small handful of miners and steelmakers which dominate both spot and contract markets. Until 2006, prices were determined in annual benchmark negotiations between the main iron ore producers ( BHP, Rio Tinto, and
Over the last 40 years, iron ore prices have been decided in closed-door negotiations between the small handful of miners and steelmakers which dominate both spot and contract markets. Until 2006, prices were determined in annual benchmark negotiations between the main iron ore producers ( BHP, Rio Tinto, and
''History of the Iron Ore Trade on the Great Lakes''
(1910 Annual Report of the Lake Carriers' Association, made available online by the Michael Schwartz Library of Cleveland State University) ** James Stephen Jeans,
Pioneers of the Cleveland Iron Trade
' (1875) * Modern information *
Global price of Iron Ore
data from the
Iron Ore Statistics and Information
from the U.S. Geological Survey's National Minerals Information Center *
World's Largest Iron Ore Producers, 2023
analysis from James F. King {{DEFAULTSORT:Iron Ore Articles containing video clips Economic geology

 Iron ores are rocks and
Iron ores are rocks and mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Mi ...
s from which metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated wit ...
lic iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
can be economically extracted. The ores are usually rich in iron oxide
An iron oxide is a chemical compound composed of iron and oxygen. Several iron oxides are recognized. Often they are non-stoichiometric. Ferric oxyhydroxides are a related class of compounds, perhaps the best known of which is rust.
Iron ...
s and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the form of magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
(, 72.4% Fe), hematite (, 69.9% Fe), goethite
Goethite (, ) is a mineral of the diaspore group, consisting of iron(III) oxide-hydroxide, specifically the α- polymorph. It is found in soil and other low-temperature environments such as sediment. Goethite has been well known since ancient t ...
(, 62.9% Fe), limonite
Limonite () is an iron ore consisting of a mixture of hydrated iron(III) oxide-hydroxides in varying composition. The generic formula is frequently written as , although this is not entirely accurate as the ratio of oxide to hydroxide can vary qu ...
(, 55% Fe), or siderite (, 48.2% Fe).
Ores containing very high quantities of hematite or magnetite (typically greater than about 60% iron) are known as natural ore or irect shipping ore and can be fed directly into iron-making blast furnaces. Iron ore is the raw material
A raw material, also known as a feedstock, unprocessed material, or primary commodity, is a basic material that is used to produce goods, finished goods, energy, or intermediate materials/Intermediate goods that are feedstock for future finished ...
used to make pig iron
Pig iron, also known as crude iron, is an intermediate good used by the iron industry in the production of steel. It is developed by smelting iron ore in a blast furnace. Pig iron has a high carbon content, typically 3.8–4.7%, along with si ...
, which is one of the main raw materials to make steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength a ...
— 98% of the mined iron ore is used to make steel. In 2011 the ''Financial Times
The ''Financial Times'' (''FT'') is a British daily newspaper printed in broadsheet and also published digitally that focuses on business and economic Current affairs (news format), current affairs. Based in London, the paper is owned by a Jap ...
'' quoted Christopher LaFemina, mining analyst at Barclays Capital, saying that iron ore is "more integral to the global economy than any other commodity, except perhaps oil".
Sources
Elemental iron is virtually absent on theEarth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
's surface except as iron-nickel alloys from meteorites and very rare forms of deep mantle xenoliths. Although iron is the fourth most abundant element in Earth's crust
Earth's crust is its thick outer shell of rock, referring to less than one percent of the planet's radius and volume. It is the top component of the lithosphere, a solidified division of Earth's layers that includes the crust and the upper ...
, composing about 5% by weight, the vast majority is bound in silicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used ...
or, more rarely, carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
minerals, and smelting pure iron from these minerals would require a prohibitive amount of energy. Therefore, all sources of iron used by human industry exploit comparatively rarer iron oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
minerals, primarily hematite.
Prehistoric societies used laterite as a source of iron ore. Prior to the industrial revolution, most iron was obtained from widely-available goethite
Goethite (, ) is a mineral of the diaspore group, consisting of iron(III) oxide-hydroxide, specifically the α- polymorph. It is found in soil and other low-temperature environments such as sediment. Goethite has been well known since ancient t ...
or bog ore, for example, during the American Revolution
The American Revolution (1765–1783) was a colonial rebellion and war of independence in which the Thirteen Colonies broke from British America, British rule to form the United States of America. The revolution culminated in the American ...
and the Napoleonic Wars
{{Infobox military conflict
, conflict = Napoleonic Wars
, partof = the French Revolutionary and Napoleonic Wars
, image = Napoleonic Wars (revision).jpg
, caption = Left to right, top to bottom:Battl ...
. Historically, much of the iron ore utilized by industrialized societies has been mined from predominantly hematite deposits with grades of around 70% Fe. These deposits are commonly referred to as "direct shipping ores" or "natural ores". Increasing iron ore demand, coupled with the depletion of high-grade hematite ores in the United States, led after World War II
World War II or the Second World War (1 September 1939 – 2 September 1945) was a World war, global conflict between two coalitions: the Allies of World War II, Allies and the Axis powers. World War II by country, Nearly all of the wo ...
to the development of lower-grade iron ore sources, principally the use of magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
and taconite.
Iron ore mining methods vary by the type of ore being mined. There are four main types of iron ore deposits worked currently, depending on the mineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical mineralogy, optical) properties of minerals and mineralized artifact (archaeology), artifacts. Specific s ...
and geology of the ore deposits. These are magnetite, titanomagnetite, hematite, and pisolitic ironstone deposits.
The origin of iron can be ultimately traced to its formation through nuclear fusion in stars, and most of the iron is thought to have originated in dying stars that are large enough to explode as supernova
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last stellar evolution, evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion ...
e. The Earth's core is thought to consist mainly of iron, but this is inaccessible from the surface. Some iron meteorites are thought to have originated from asteroids
An asteroid is a minor planet—an object larger than a meteoroid that is neither a planet nor an identified comet—that orbits within the Solar System#Inner Solar System, inner Solar System or is co-orbital with Jupiter (Trojan asteroids). As ...
in diameter or larger.
Banded iron formations
Banded iron formations (BIFs) aresedimentary rock
Sedimentary rocks are types of rock (geology), rock formed by the cementation (geology), cementation of sediments—i.e. particles made of minerals (geological detritus) or organic matter (biological detritus)—that have been accumulated or de ...
s containing more than 15% iron composed predominantly of thinly-bedded iron minerals and silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
(as quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
). Banded iron formations occur exclusively in Precambrian rocks, and are commonly weakly-to-intensely metamorphosed. Banded iron formations may contain iron in carbonates ( siderite or ankerite) or silicates
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for an ...
(minnesotaite
Minnesotaite is an iron silicate mineral with formula: (Fe2+,Mg)3Si4O10(OH)2. It crystallizes in the triclinic crystal system and occurs as fine needles and platelets with other silicates. It is isostructural with the pyrophyllite-talc mineral g ...
, greenalite, or grunerite), but in those mined as iron ores, oxides (magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
or hematite) are the principal iron mineral. Banded iron formations are known as '' taconite'' within North America.
The mining involves moving tremendous amounts of ore and waste. The waste comes in two forms: non-ore bedrock in the mine ( overburden or interburden locally known as mullock), and unwanted minerals, which are an intrinsic part of the ore rock itself ( gangue). The mullock is mined and piled in waste dumps, and the gangue is separated during the beneficiation process and is removed as tailings. Taconite tailings are mostly the mineral quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
, which is chemically inert. This material is stored in large, regulated water settling ponds.
Magnetite ores
The key parameters for magnetite ore being economic are the crystallinity of the magnetite, the grade of the iron within the banded iron formation host rock, and the contaminant elements which exist within the magnetite concentrate. The size and strip ratio of most magnetite resources is irrelevant, as a banded iron formation can be hundreds of meters thick, extend hundreds of kilometers along strike, and can easily come to more than three billion or more tonnes of contained ore. The typical grade of iron at which a magnetite-bearing banded iron formation becomes economic is roughly 25% iron, which can generally yield a 33% to 40% recovery of magnetite by weight, to produce a concentrate grading in excess of 64% iron by weight. The typical magnetite iron ore concentrate has less than 0.1%phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
, 3–7% silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
, and less than 3% aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
.
As of 2019, magnetite iron ore is mined in Minnesota
Minnesota ( ) is a U.S. state, state in the Upper Midwestern region of the United States. It is bordered by the Canadian provinces of Manitoba and Ontario to the north and east and by the U.S. states of Wisconsin to the east, Iowa to the so ...
and Michigan
Michigan ( ) is a peninsular U.S. state, state in the Great Lakes region, Great Lakes region of the Upper Midwest, Upper Midwestern United States. It shares water and land boundaries with Minnesota to the northwest, Wisconsin to the west, ...
in the United States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
, eastern Canada
Canada is a country in North America. Its Provinces and territories of Canada, ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, making it the world's List of coun ...
, and northern Sweden
Sweden, formally the Kingdom of Sweden, is a Nordic countries, Nordic country located on the Scandinavian Peninsula in Northern Europe. It borders Norway to the west and north, and Finland to the east. At , Sweden is the largest Nordic count ...
. Magnetite-bearing banded iron formation is mined extensively in Brazil
Brazil, officially the Federative Republic of Brazil, is the largest country in South America. It is the world's List of countries and dependencies by area, fifth-largest country by area and the List of countries and dependencies by population ...
as of 2019, which exports significant quantities to Asia
Asia ( , ) is the largest continent in the world by both land area and population. It covers an area of more than 44 million square kilometres, about 30% of Earth's total land area and 8% of Earth's total surface area. The continent, which ...
, and there is a nascent and large magnetite iron ore industry in Australia
Australia, officially the Commonwealth of Australia, is a country comprising mainland Australia, the mainland of the Australia (continent), Australian continent, the island of Tasmania and list of islands of Australia, numerous smaller isl ...
.
Direct-shipping (hematite) ores
Direct-shipping iron ore (DSO) deposits (typically composed of hematite) are currently exploited on all continents exceptAntarctica
Antarctica () is Earth's southernmost and least-populated continent. Situated almost entirely south of the Antarctic Circle and surrounded by the Southern Ocean (also known as the Antarctic Ocean), it contains the geographic South Pole. ...
, with the largest intensity in South America
South America is a continent entirely in the Western Hemisphere and mostly in the Southern Hemisphere, with a considerably smaller portion in the Northern Hemisphere. It can also be described as the southern Subregion#Americas, subregion o ...
, Australia, and Asia. Most large hematite iron ore deposits are sourced from altered banded iron formations and (rarely) igneous accumulations.
DSO deposits are typically rarer than the magnetite-bearing BIF or other rocks which form its main source, or protolith rock, but are considerably cheaper to mine and process as they require less beneficiation due to the higher iron content. However, DSO ores can contain significantly higher concentrations of penalty elements, typically being higher in phosphorus, water content (especially pisolite sedimentary accumulations), and aluminium ( clays within pisolites). Export-grade DSO ores are generally in the 62–64% Fe range.
Magmatic magnetite ore deposits
Granite
Granite ( ) is a coarse-grained (phanerite, phaneritic) intrusive rock, intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a high content of silica and alkali metal oxides that slowly coo ...
and ultrapotassic igneous rocks were sometimes used to segregate magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
crystals and form masses of magnetite suitable for economic concentration. A few iron ore deposits, notably in Chile
Chile, officially the Republic of Chile, is a country in western South America. It is the southernmost country in the world and the closest to Antarctica, stretching along a narrow strip of land between the Andes, Andes Mountains and the Paci ...
, are formed from volcanic flows containing significant accumulations of magnetite phenocrysts.
Mine tailings
For every one ton of iron ore concentrate produced, approximately 2.5–3.0 tons of iron ore tailings will be discharged. Statistics show that there are 130 million tons of iron ore tailings discharged every year. If, for example, the mine tailings contain an average of approximately 11% iron, there would be approximately 1.41 million tons of iron wasted annually. These tailings are also high in other useful metals such ascopper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
, nickel, and cobalt, and they can be used for road-building materials like pavement and filler and building materials such as cement, low-grade glass, and wall materials. While tailings are a relatively low-grade ore, they are also inexpensive to collect, as they do not have to be mined. Because of this, companies such as Magnetation have started reclamation projects where they use iron ore tailings as a source of metallic iron.
The two main methods of recycling iron from iron ore tailings are magnetizing roasting and direct reduction. Magnetizing roasting uses temperatures between for a time of under 1 hour to produce an iron concentrate (Fe3O4) to be used for iron smelting. For magnetizing roasting, it is important to have a reducing atmosphere to prevent oxidization and the formation of Fe2O3 because it is harder to separate as it is less magnetic. Direct reduction uses hotter temperatures of over and longer times of 2–5 hours. Direct reduction is used to produce sponge iron (Fe) to be used for steel-making. Direct reduction requires more energy, as the temperatures are higher and the time is longer and it requires more reducing agent than magnetizing roasting.
Extraction
Lower-grade sources of iron ore generally require beneficiation, using techniques like crushing, milling, gravity or heavy media separation, screening, and silica froth flotation to improve the concentration of the ore and remove impurities. The results, high-quality fine ore powders, are known as fines.Magnetite
Magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
is magnetic, and hence easily separated from the gangue minerals and capable of producing a high-grade concentrate with very low levels of impurities.
The grain size of the magnetite and its degree of commingling with the silica groundmass determine the grind size to which the rock must be comminuted to enable efficient magnetic separation to provide a high-purity magnetite concentrate. This determines the energy inputs required to run a milling operation.
Mining of banded iron formations involves coarse crushing and screening, followed by rough crushing and fine grinding to comminute the ore to the point where the crystallized magnetite and quartz are fine enough that the quartz is left behind when the resultant powder is passed under a magnetic separator.
Generally, most magnetite banded iron formation deposits must be ground to between in order to produce a low-silica magnetite concentrate. Magnetite concentrate grades are generally in excess of 70% iron by weight and usually are low in phosphorus, aluminium, titanium, and silica and demand a premium price.
Hematite
Due to the highdensity
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be u ...
of hematite relative to associated silicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used ...
gangue, hematite beneficiation usually involves a combination of beneficiation techniques. One method relies on passing the finely-crushed ore over a slurry containing magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
or other agent such as ferrosilicon
Ferrosilicon is an ferroalloy, alloy of iron and silicon. It has a typical silicon content of 15–90% by weight and a high proportion of iron silicides.
Production and reactions
Ferrosilicon is produced by reduction of silica or sand with coke ...
which increases its density. When the density of the slurry is properly calibrated, the hematite will sink and the silicate mineral fragments will float and can be removed.
Production and consumption
''Financial Times'', October 26, 2009 Iron-rich rocks are common worldwide, but ore-grade commercial
mining
Mining is the Resource extraction, extraction of valuable geological materials and minerals from the surface of the Earth. Mining is required to obtain most materials that cannot be grown through agriculture, agricultural processes, or feasib ...
operations are dominated by the countries listed in the table aside. The major constraint to economics for iron ore deposits is not necessarily the grade or size of the deposits, because it is not particularly hard to geologically prove enough tonnage of the rocks exist. The main constraint is the position of the iron ore relative to market, the cost of rail infrastructure to get it to market, and the energy cost required to do so.
Mining iron ore is a high-volume, low-margin business, as the value of iron is significantly lower than base metals.Iron ore pricing war''Financial Times'', October 14, 2009 It is highly capital intensive, and requires significant investment in infrastructure such as rail in order to transport the ore from the mine to a freight ship. For these reasons, iron ore production is concentrated in the hands of a few major players. World production averages of raw ore annually. The world's largest producer of iron ore is the Brazilian mining corporation
Vale
A vale is a type of valley.
Vale may also refer to:
Places Georgia
* Vale, Georgia, a town in the Samtskhe-Javakheti region
Norway
* Våle, a historic municipality
Portugal
* Vale (Santa Maria da Feira), a former civil parish in the municip ...
, followed by Australian companies Rio Tinto and BHP. A further Australian supplier, Fortescue, has helped bring Australia's production to first in the world.
The seaborne trade in iron ore—that is, iron ore to be shipped to other countries—was in 2004. Australia and Brazil dominate the seaborne trade, with 72% of the market. BHP, Rio and Vale control 66% of this market between them.
In Australia
Australia, officially the Commonwealth of Australia, is a country comprising mainland Australia, the mainland of the Australia (continent), Australian continent, the island of Tasmania and list of islands of Australia, numerous smaller isl ...
, iron ore is won from three main sources: pisolite " channel iron deposit" ore derived by mechanical erosion of primary banded-iron formations and accumulated in alluvial channels such as at Pannawonica; and the dominant metasomatically altered banded iron formation-related ores such as at Newman, the Chichester Range, the Hamersley Range
The Hamersley Range is a mountainous region of the Pilbara region of Western Australia. The range was named on 12 June 1861 by explorer Francis Thomas Gregory after Edward Hamersley, a prominent promoter of his exploration expedition to the ...
and Koolyanobbing, Western Australia
Western Australia (WA) is the westernmost state of Australia. It is bounded by the Indian Ocean to the north and west, the Southern Ocean to the south, the Northern Territory to the north-east, and South Australia to the south-east. Western Aust ...
. Other types of ore are coming to the fore recently, such as oxidised ferruginous hardcaps, for instance laterite iron ore deposits near Lake Argyle in Western Australia.
The total recoverable reserves of iron ore in India
India, officially the Republic of India, is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area; the List of countries by population (United Nations), most populous country since ...
are about of hematite and of magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
. Chhattisgarh, Madhya Pradesh
Madhya Pradesh (; ; ) is a state in central India. Its capital is Bhopal and the largest city is Indore, Indore. Other major cities includes Gwalior, Jabalpur, and Sagar, Madhya Pradesh, Sagar. Madhya Pradesh is the List of states and union te ...
, Karnataka
Karnataka ( ) is a States and union territories of India, state in the southwestern region of India. It was Unification of Karnataka, formed as Mysore State on 1 November 1956, with the passage of the States Reorganisation Act, 1956, States Re ...
, Jharkhand
Jharkhand (; ) is a States and union territories of India, state in East India, eastern India. The state shares its border with the states of West Bengal to the east, Chhattisgarh to the west, Uttar Pradesh to the northwest, Bihar to the north ...
, Odisha
Odisha (), formerly Orissa (List of renamed places in India, the official name until 2011), is a States and union territories of India, state located in East India, Eastern India. It is the List of states and union territories of India by ar ...
, Goa, Maharashtra
Maharashtra () is a state in the western peninsular region of India occupying a substantial portion of the Deccan Plateau. It is bordered by the Arabian Sea to the west, the Indian states of Karnataka and Goa to the south, Telangana to th ...
, Andhra Pradesh
Andhra Pradesh (ISO 15919, ISO: , , AP) is a States and union territories of India, state on the East Coast of India, east coast of southern India. It is the List of states and union territories of India by area, seventh-largest state and th ...
, Kerala
Kerala ( , ) is a States and union territories of India, state on the Malabar Coast of India. It was formed on 1 November 1956, following the passage of the States Reorganisation Act, by combining Malayalam-speaking regions of the erstwhile ...
, Rajasthan
Rajasthan (; Literal translation, lit. 'Land of Kings') is a States and union territories of India, state in northwestern India. It covers or 10.4 per cent of India's total geographical area. It is the List of states and union territories of ...
, and Tamil Nadu
Tamil Nadu (; , TN) is the southernmost States and union territories of India, state of India. The List of states and union territories of India by area, tenth largest Indian state by area and the List of states and union territories of Indi ...
are the principal Indian producers of iron ore. World consumption of iron ore grows 10% per year on average with the main consumers being China, Japan, Korea, the United States, and the European Union.
China is currently the largest consumer of iron ore, which translates to be the world's largest steel producing country. It is also the largest importer, buying 52% of the seaborne trade in iron ore in 2004. China is followed by Japan and Korea, which consume a significant amount of raw iron ore and metallurgical coal. In 2006, China produced of iron ore, with an annual growth of 38%.
Iron ore market

 Over the last 40 years, iron ore prices have been decided in closed-door negotiations between the small handful of miners and steelmakers which dominate both spot and contract markets. Until 2006, prices were determined in annual benchmark negotiations between the main iron ore producers ( BHP, Rio Tinto, and
Over the last 40 years, iron ore prices have been decided in closed-door negotiations between the small handful of miners and steelmakers which dominate both spot and contract markets. Until 2006, prices were determined in annual benchmark negotiations between the main iron ore producers ( BHP, Rio Tinto, and Vale
A vale is a type of valley.
Vale may also refer to:
Places Georgia
* Vale, Georgia, a town in the Samtskhe-Javakheti region
Norway
* Våle, a historic municipality
Portugal
* Vale (Santa Maria da Feira), a former civil parish in the municip ...
) and Japanese importers. In 2006, Chinese company Baosteel began handling negotiations for the importer side. The Chinese government replaced Baosteel with China Iron and Steel Association as lead negotiator in 2009. Traditionally, the first deal reached between these the major producers and the major importers sets a benchmark to be followed by the rest of the industry.
Singapore Mercantile Exchange (SMX) has launched the world's first global iron ore futures contract, based on the Metal Bulletin Iron Ore Index (MBIOI) which uses daily price data from a broad spectrum of industry participants and independent Chinese steel consultancy and data provider Shanghai Steelhome's widespread contact base of steel producers and iron ore traders across China. The futures contract has seen monthly volumes over after eight months of trading.
This move follows a switch to index-based quarterly pricing by the world's three largest iron ore miners—Vale
A vale is a type of valley.
Vale may also refer to:
Places Georgia
* Vale, Georgia, a town in the Samtskhe-Javakheti region
Norway
* Våle, a historic municipality
Portugal
* Vale (Santa Maria da Feira), a former civil parish in the municip ...
, Rio Tinto, and BHP—in early 2010, breaking a 40-year tradition of benchmark annual pricing.
Abundance by country
Available world iron ore resources
Iron is the most abundant element on earth but not in the crust. The extent of the accessible iron ore reserves is not known, though Lester Brown of the Worldwatch Institute suggested in 2006 that iron ore could run out within 64 years (that is, by 2070), based on 2% growth in demand per year.Australia
Geoscience Australia calculates that the country's " economic demonstrated resources" of iron currently amount to 24 gigatonnes, or . Another estimate places Australia's reserves of iron ore at , or 30% of the world's estimated , of which Western Australia accounts for . The current production rate from the Pilbara region ofWestern Australia
Western Australia (WA) is the westernmost state of Australia. It is bounded by the Indian Ocean to the north and west, the Southern Ocean to the south, the Northern Territory to the north-east, and South Australia to the south-east. Western Aust ...
is approximately per year and rising. Gavin Mudd ( RMIT University) and Jonathon Law ( CSIRO) expect it to be gone within 30–50 years and 56 years, respectively. These 2010 estimates require ongoing review to take into account shifting demand for lower-grade iron ore and improving mining and recovery techniques (allowing deeper mining below the groundwater table).
Brazil
Brazil
Brazil, officially the Federative Republic of Brazil, is the largest country in South America. It is the world's List of countries and dependencies by area, fifth-largest country by area and the List of countries and dependencies by population ...
is the second-largest producer of iron ore after Australia, accounting for 16% of the world's iron ore production. After a somewhat sluggish production volume 2010-2020, partly due to the Mariana dam disaster in 2015 and the Brumadinho dam disaster in 2019, which halted the production at the two involved mines, production has increased steadily since 2021, when Brazil produced . In 2022 it increased to and in 2023 to .
The Brazilian production is expected to rise by a CAGR of 2% between 2023 and 2027, and industry analyst Fitch Solutions forecasted in 2021 that Brazil's annual production will reach by 2030.
Canada
In 2017, Canadian iron ore mines produced of iron ore in concentrate pellets and 13.6 million tons of crude steel. Of the of steel was exported, and of iron ore was exported at a value of $4.6 billion. Of the iron ore exported, 38.5% of the volume was iron ore pellets with a value of $2.3 billion, and 61.5% was iron ore concentrates with a value of $2.3 billion. 46% of Canada's iron ore comes from the Iron Ore Company of Canada mine, in Labrador City,Newfoundland
Newfoundland and Labrador is the easternmost province of Canada, in the country's Atlantic region. The province comprises the island of Newfoundland and the continental region of Labrador, having a total size of . As of 2025 the population ...
, with secondary sources including the Mary River Mine in Nunavut
Nunavut is the largest and northernmost Provinces and territories of Canada#Territories, territory of Canada. It was separated officially from the Northwest Territories on April 1, 1999, via the ''Nunavut Act'' and the Nunavut Land Claims Agr ...
.
India
According to the U.S. Geological Survey's 2021 Report on iron ore, India is estimated to produce of iron ore in 2020, placing it as the seventh-largest global center of iron ore production, behind Australia, Brazil, China, Russia, South Africa, and Ukraine. India's iron ore production in 2023 was 285,000,000 metric tonnes and was the fourth largest producer in the world.Ukraine
According to the U.S. Geological Survey's 2021 report on iron ore, Ukraine is estimated to have produced of iron ore in 2020, placing it as the seventh largest global center of iron ore production, behind Australia, Brazil, China, India, Russia, and South Africa. Producers of iron ore in Ukraine include Ferrexpo, Metinvest, and ArcelorMittal Kryvyi Rih.United States
In 2014, mines in theUnited States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
produced of iron ore with an estimated value of $5.1 billion. Iron mining in the United States is estimated to have accounted for 2% of the world's iron ore output. In the United States there are twelve iron ore mines, with nine being open pit mines and three being reclamation operations. There were also ten pelletizing plants, nine concentration plants, two direct-reduced iron (DRI) plants, and one iron nugget plant that were operating in 2014. In the United States the majority of iron ore mining is in the iron ranges around Lake Superior. These iron ranges occur in Minnesota
Minnesota ( ) is a U.S. state, state in the Upper Midwestern region of the United States. It is bordered by the Canadian provinces of Manitoba and Ontario to the north and east and by the U.S. states of Wisconsin to the east, Iowa to the so ...
and Michigan, which combined accounted for 93% of the usable iron ore produced in the United States in 2014. Seven of the nine operational open pit mines in the United States are located in Minnesota as well as two of the three tailings reclamation operations. The other two active open pit mines were located in Michigan
Michigan ( ) is a peninsular U.S. state, state in the Great Lakes region, Great Lakes region of the Upper Midwest, Upper Midwestern United States. It shares water and land boundaries with Minnesota to the northwest, Wisconsin to the west, ...
. In 2016, one of the two mines shut down. There have also been iron ore mines in Utah
Utah is a landlocked state in the Mountain states, Mountain West subregion of the Western United States. It is one of the Four Corners states, sharing a border with Arizona, Colorado, and New Mexico. It also borders Wyoming to the northea ...
and Alabama
Alabama ( ) is a U.S. state, state in the Southeastern United States, Southeastern and Deep South, Deep Southern regions of the United States. It borders Tennessee to the north, Georgia (U.S. state), Georgia to the east, Florida and the Gu ...
; however, the last iron ore mine in Utah shut down in 2014 and the last iron ore mine in Alabama shut down in 1975.Lewis S. Dean, Minerals in the economy of Alabama 2007Archived 2015-09-24 at the Wayback Machine
The Wayback Machine is a digital archive of the World Wide Web founded by Internet Archive, an American nonprofit organization based in San Francisco, California. Launched for public access in 2001, the service allows users to go "back in ...
, Alabama Geological Survey, 2008
Smelting
Iron ores consist ofoxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
and iron atoms bonded together into molecules. To convert it to metallic iron, it must be smelted or sent through a direct reduction process to remove the oxygen. Oxygen-iron bonds are strong, and to remove the iron from the oxygen, a stronger elemental bond must be presented to attach to the oxygen. Carbon is used because the strength of a carbon-oxygen bond is greater than that of the iron-oxygen bond at high temperatures. Thus, the iron ore must be powdered and mixed with coke, to be burnt in the smelting process.
Carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
is the primary ingredient of chemically stripping oxygen from iron. Thus, the iron and carbon smelting must be kept in an oxygen-deficient (reducing) state to promote the burning of carbon to produce and not .
* Air blast and charcoal (coke): 2 C + O2 → 2 CO
* Carbon monoxide (CO) is the principal reduction agent.
** Stage One: 3 Fe2O3 + CO → 2 Fe3O4 + CO2
** Stage Two: Fe3O4 + CO → 3 FeO + CO2
** Stage Three: FeO + CO → Fe + CO2
* Limestone calcining: CaCO3 → CaO + CO2
* Lime acting as flux: CaO + SiO2 → CaSiO3
Trace elements
The inclusion of even small amounts of some elements can have profound effects on the behavioral characteristics of a batch of iron or the operation of a smelter. These effects can be both good and bad, some catastrophically bad. Some chemicals are deliberately added, such as flux, which makes a blast furnace more efficient. Others are added because they make the iron more fluid, harder, or give it some other desirable quality. The choice of ore, fuel, and flux determines how the slag behaves and the operational characteristics of the iron produced. Ideally, iron ore contains only iron and oxygen. In reality, this is rarely the case. Typically, iron ore contains a host of elements which are often unwanted in modern steel.Silicon
Silica () is almost always present in iron ore. Most of it is slagged off during the smelting process. At temperatures above , some will be reduced and form an alloy with the iron. The hotter the furnace, the more silicon will be present in the iron. It is not uncommon to find up to 1.5% Si in European cast iron from the 16th to 18th centuries. The major effect of silicon is to promote the formation of grey iron. Grey iron is less brittle and easier to finish than white iron. It is preferred for casting purposes for this reason. British metallurgist Thomas Turner reported that silicon also reduces shrinkage and the formation of blowholes, lowering the number of bad castings. However, too much silicon present in the iron leads to increased brittleness and moderate hardness.Phosphorus
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
(P) has four major effects on iron: increased hardness and strength, lower solidus, increased fluidity, and cold shortness. Depending on the use intended for the iron, these effects are either good or bad. Bog ore often has a high phosphorus content.
The strength and hardness of iron increases with the concentration of phosphorus. 0.05% phosphorus in wrought iron makes it as hard as medium-carbon steel. High-phosphorus iron can also be hardened by cold hammering. The hardening effect is true for any concentration of phosphorus. The more phosphorus, the harder the iron becomes and the more it can be hardened by hammering. Modern steel makers can increase hardness by as much as 30%, without sacrificing shock resistance by maintaining phosphorus levels between 0.07 and 0.12%. It also increases the depth of hardening due to quenching, but at the same time also decreases the solubility of carbon in iron at high temperatures. This would decrease its usefulness in making blister steel ( cementation), where the speed and amount of carbon absorption is the overriding consideration.
The addition of phosphorus has a downside. At concentrations higher than 0.2%, iron becomes increasingly cold short, or brittle at low temperatures. Cold short is especially important for bar iron. Although bar iron is usually worked hot, its uses often require it to be tough, bendable, and resistant to shock at room temperature. A nail that shatters when hit with a hammer or a carriage wheel that breaks when it hit a rock would not sell well. High enough concentrations of phosphorus render any iron unusable. The effects of cold shortness are magnified by temperature. Thus, a piece of iron that is perfectly serviceable in summer might become extremely brittle in winter. There is some evidence that during the Middle Ages
In the history of Europe, the Middle Ages or medieval period lasted approximately from the 5th to the late 15th centuries, similarly to the post-classical period of global history. It began with the fall of the Western Roman Empire and ...
the very wealthy may have had a high-phosphorus sword for summer and a low-phosphorus sword for winter.
Careful control of phosphorus can be of great benefit in casting operations. Phosphorus depresses the liquidus, allowing the iron to remain molten for longer and increasing fluidity. The addition of 1% can double the distance molten iron will flow. The maximum effect, about , is achieved at a concentration of 10.2%. For foundry work Turner felt the ideal iron had 0.2–0.55% phosphorus. The resulting iron filled molds with fewer voids and also shrank less. In the 19th century some producers of decorative cast iron used iron with up to 5% phosphorus. The extreme fluidity allowed them to make very complex and delicate castings, but they could not be weight-bearing, as they had no strength.
There are two remedies for high-phosphorus iron. The oldest, easiest, and cheapest, is avoidance. If the iron that the ore produced was cold short, one would search for a new source of iron ore. The second method involves oxidizing the phosphorus during the fining process by adding iron oxide. This technique is usually associated with puddling in the 19th century, and may not have been understood earlier. For instance, Isaac Zane, owner of Marlboro Iron Works, did not appear to know about it in 1772. Given Zane's reputation for keeping abreast of the latest developments, the technique was probably unknown to the ironmasters of Virginia
Virginia, officially the Commonwealth of Virginia, is a U.S. state, state in the Southeastern United States, Southeastern and Mid-Atlantic (United States), Mid-Atlantic regions of the United States between the East Coast of the United States ...
and Pennsylvania
Pennsylvania, officially the Commonwealth of Pennsylvania, is a U.S. state, state spanning the Mid-Atlantic (United States), Mid-Atlantic, Northeastern United States, Northeastern, Appalachian, and Great Lakes region, Great Lakes regions o ...
.
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
is generally considered to be a deleterious contaminant because it makes steel brittle, even at concentrations of as little as 0.6%. When the Gilchrist–Thomas process allowed the removal of bulk amounts of the element from cast iron in the 1870s, it was a major development because most of the iron ores mined in continental Europe at the time were phosphorous. However, removing all the contaminant by fluxing or smelting is complicated, and so desirable iron ores must generally be low in phosphorus to begin with.
Aluminium
Small amounts ofaluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
(Al) are present in many ores including iron ore, sand, and some limestones. The former can be removed by washing the ore prior to smelting. Until the introduction of brick-lined furnaces, the amount of aluminium contamination was small enough that it did not have an effect on either the iron or slag. However, when brick began to be used for hearths and the interior of blast furnaces, the amount of aluminium contamination increased dramatically. This was due to the erosion of the furnace lining by the liquid slag.
Aluminium is difficult to reduce. As a result, aluminium contamination of the iron is not a problem. However, it does increase the viscosity of the slag. This will have a number of adverse effects on furnace operation. The thicker slag will slow the descent of the charge, prolonging the process. High aluminium will also make it more difficult to tap off the liquid slag. At the extreme, this could lead to a frozen furnace.
There are a number of solutions to a high-aluminium slag. The first is avoidance; do not use ore or a lime source with a high aluminium content. Increasing the ratio of lime flux will decrease the viscosity.
Sulfur
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
(S) is a frequent contaminant in coal. It is also present in small quantities in many ores, but can be removed by calcining. Sulfur dissolves readily in both liquid and solid iron at the temperatures present in iron smelting. The effects of even small amounts of sulfur are immediate and serious. They were one of the first worked out by iron makers. Sulfur causes iron to be red or hot short.
Hot short iron is brittle when hot. This was a serious problem as most iron used during the 17th and 18th centuries was bar or wrought iron. Wrought iron is shaped by repeated blows with a hammer while hot. A piece of hot short iron will crack if worked with a hammer. When a piece of hot iron or steel cracks, the exposed surface immediately oxidizes. This layer of oxide prevents the mending of the crack by welding. Large cracks cause the iron or steel to break up. Smaller cracks can cause the object to fail during use. The degree of hot shortness is in direct proportion to the amount of sulfur present. Today, iron with over 0.03% sulfur is avoided.
Hot short iron can be worked, but it must be worked at low temperatures. Working at lower temperatures requires more physical effort from the smith or forgeman. The metal must be struck more often and harder to achieve the same result. A mildly sulfur-contaminated bar can be worked, but it requires a great deal more time and effort.
In cast iron, sulfur promotes the formation of white iron. As little as 0.5% can counteract the effects of slow cooling and a high silicon content. White cast iron is more brittle, but also harder. It is generally avoided, because it is difficult to work, except in China where high-sulfur cast iron, some as high as 0.57%, made with coal and coke, was used to make bells and chimes. According to , good foundry iron should have less than 0.15% sulfur. In the rest of the world, a high-sulfur cast iron can be used for making castings, but will make poor wrought iron.
There are a number of remedies for sulfur contamination. The first, and the one most used in historic and prehistoric operations, is avoidance. Coal was not used in Europe (unlike China) as a fuel for smelting because it contains sulfur and therefore causes hot short iron. If an ore resulted in hot short metal, ironmaster
An ironmaster is the manager, and usually owner, of a forge or blast furnace for the processing of iron. It is a term mainly associated with the period of the Industrial Revolution, especially in Great Britain.
The ironmaster was usually a larg ...
s looked for another ore. When mineral coal was first used in European blast furnaces in 1709 (or perhaps earlier), it was coked. Only with the introduction of hot blast from 1829 was raw coal used.
Ore roasting
Sulfur can be removed from ores by roasting and washing. Roasting oxidizes sulfur to formsulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
(SO2), which either escapes into the atmosphere or can be washed out. In warm climates, it is possible to leave pyritic ore out in the rain. The combined action of rain, bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
, and heat oxidize the sulfides to sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
and sulfates, which are water-soluble and leached out. However, historically (at least), iron sulfide (iron pyrite ), though a common iron mineral, has not been used as an ore for the production of iron metal. Natural weathering was also used in Sweden. The same process, at geological speed, results in the gossan limonite
Limonite () is an iron ore consisting of a mixture of hydrated iron(III) oxide-hydroxides in varying composition. The generic formula is frequently written as , although this is not entirely accurate as the ratio of oxide to hydroxide can vary qu ...
ores.
The importance attached to low-sulfur iron is demonstrated by the consistently higher prices paid for the iron of Sweden, Russia, and Spain from the 16th to 18th centuries. Today sulfur is no longer a problem. The modern remedy is the addition of manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
, but the operator must know how much sulfur is in the iron because at least five times as much manganese must be added to neutralize it. Some historic irons display manganese levels, but most are well below the level needed to neutralize sulfur.
Sulfide inclusion as manganese sulfide (MnS) can also be the cause of severe pitting corrosion problems in low-grade stainless steel
Stainless steel, also known as inox, corrosion-resistant steel (CRES), or rustless steel, is an iron-based alloy that contains chromium, making it resistant to rust and corrosion. Stainless steel's resistance to corrosion comes from its chromi ...
such as AISI 304 steel. Under oxidizing conditions and in the presence of moisture, when sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
oxidizes, it produces thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
anions as intermediate species, and because the thiosulfate anion has a higher equivalent electromobility than the chloride anion due to its double negative electrical charge, it promotes pit growth. Indeed, the positive electrical charges born by Fe2+ cations released in solution by Fe oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
on the anodic zone inside the pit must be quickly compensated / neutralized by negative charges brought by the electrokinetic migration of anions in the capillary pit. Some of the electrochemical processes occurring in a capillary pit are the same as those encountered in capillary electrophoresis. The higher the anion electrokinetic migration rate, the higher the rate of pitting corrosion. Electrokinetic transport of ions inside the pit can be the rate-limiting step in the pit growth rate.
See also
* Bog iron * Ironstone * Environmental impact of iron ore miningNotes
Citations
General and cited references
* * * Ramanaidou, E. R. and Wells, M. A. (2014). 13.13 "Sedimentary Hosted Iron Ores". In: Holland, H. D. and Turekian, K. K. Eds., ''Treatise on Geochemistry'' (Second Edition). Oxford: Elsevier. 313–355. . * * * *External links
* Historical documents *''History of the Iron Ore Trade on the Great Lakes''
(1910 Annual Report of the Lake Carriers' Association, made available online by the Michael Schwartz Library of Cleveland State University) ** James Stephen Jeans,
Pioneers of the Cleveland Iron Trade
' (1875) * Modern information *
Global price of Iron Ore
data from the
International Monetary Fund
The International Monetary Fund (IMF) is a major financial agency of the United Nations, and an international financial institution funded by 191 member countries, with headquarters in Washington, D.C. It is regarded as the global lender of las ...
, made available via Federal Reserve Economic Data
Federal Reserve Economic Data (FRED) is a database maintained by the Research division of the Federal Reserve Bank of St. Louis that has more than 816,000 economic time series from various sources. They cover banking, business/fiscal, consumer p ...
*Iron Ore Statistics and Information
from the U.S. Geological Survey's National Minerals Information Center *
World's Largest Iron Ore Producers, 2023
analysis from James F. King {{DEFAULTSORT:Iron Ore Articles containing video clips Economic geology