Iron Export on:
[Wikipedia]
[Google]
[Amazon]
 Human iron metabolism is the set of chemical reactions that maintain
Human iron metabolism is the set of chemical reactions that maintain
 Most well-nourished people in industrialized countries have 4 to 5 grams of iron in their bodies (~38 mg iron/kg body weight for women and ~50 mg iron/kg body for men). Of this, about is contained in the hemoglobin needed to carry oxygen through the blood (around 0.5 mg of iron per mL of blood), and most of the rest (approximately 2 grams in adult men, and somewhat less in women of childbearing age) is contained in
Most well-nourished people in industrialized countries have 4 to 5 grams of iron in their bodies (~38 mg iron/kg body weight for women and ~50 mg iron/kg body for men). Of this, about is contained in the hemoglobin needed to carry oxygen through the blood (around 0.5 mg of iron per mL of blood), and most of the rest (approximately 2 grams in adult men, and somewhat less in women of childbearing age) is contained in
 Human iron homeostasis is regulated at two different levels. Systemic iron levels are balanced by the controlled absorption of dietary iron by
Human iron homeostasis is regulated at two different levels. Systemic iron levels are balanced by the controlled absorption of dietary iron by

 The expression of hepcidin, which only occurs in certain cell types such as
The expression of hepcidin, which only occurs in certain cell types such as
 Functional or actual iron deficiency can result from a variety of causes. These causes can be grouped into several categories:
* Increased demand for iron, which the diet cannot accommodate.
* Increased loss of iron (usually through loss of blood).
* Nutritional deficiency. This can result due to a lack of dietary iron or consumption of foods that inhibit iron absorption. Absorption inhibition has been observed caused by
Functional or actual iron deficiency can result from a variety of causes. These causes can be grouped into several categories:
* Increased demand for iron, which the diet cannot accommodate.
* Increased loss of iron (usually through loss of blood).
* Nutritional deficiency. This can result due to a lack of dietary iron or consumption of foods that inhibit iron absorption. Absorption inhibition has been observed caused by
A comprehensive NIH factsheet on iron and nutrition
Iron Disorders Institute: A nonprofit group concerned with iron disorders; site has helpful links and information on iron-related medical disorders.
An interactive medical learning portal on iron metabolism
Information about iron outside the body
{{Authority control Hematology Human homeostasis Biology and pharmacology of chemical elements
 Human iron metabolism is the set of chemical reactions that maintain
Human iron metabolism is the set of chemical reactions that maintain human homeostasis
In biology, homeostasis (British English, British also homoeostasis; ) is the state of steady internal physics, physical and chemistry, chemical conditions maintained by organism, living systems. This is the condition of optimal functioning fo ...
of iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
at the systemic and cellular level. Iron is both necessary to the body and potentially toxic. Controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologist
Hematology ( spelled haematology in British English) is the branch of medicine concerned with the study of the cause, prognosis, treatment, and prevention of diseases related to blood. It involves treating diseases that affect the production ...
s have been especially interested in systemic iron metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
, because iron is essential for red blood cells
Red blood cells (RBCs), referred to as erythrocytes (, with -''cyte'' translated as 'cell' in modern usage) in academia and medical publishing, also known as red cells, erythroid cells, and rarely haematids, are the most common type of blood cel ...
, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload
Iron overload is the abnormal and increased accumulation of total iron in the body, leading to organ damage. The primary mechanism of organ damage is oxidative stress, as elevated intracellular iron levels increase free radical formation via the ...
, such as hereditary hemochromatosis
Hereditary haemochromatosis type 1 (HFE-related haemochromatosis) is a genetic disorder characterized by excessive intestinal absorption of Human iron metabolism, dietary iron, resulting in a pathological increase in total body iron stores. Huma ...
, and iron deficiency
Iron deficiency, or sideropenia, is the state in which a body lacks enough iron to supply its needs. Iron is present in all cells in the human body and has several vital functions, such as carrying oxygen to the tissues from the lungs as a key ...
, such as iron-deficiency anemia
Iron-deficiency anemia is anemia caused by a iron deficiency, lack of iron. Anemia is defined as a decrease in the number of red blood cells or the amount of hemoglobin in the blood. When onset is slow, symptoms are often vague such as Fatigue ( ...
.
Importance of iron regulation
Iron is an essential bioelement for most forms of life, frombacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
to mammals
A mammal () is a vertebrate animal of the class Mammalia (). Mammals are characterised by the presence of milk-producing mammary glands for feeding their young, a broad neocortex region of the brain, fur or hair, and three middle e ...
. Its importance lies in its ability to mediate electron transfer. In the ferrous state (Fe2+), iron acts as an electron donor
In chemistry, an electron donor is a chemical entity that transfers electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process. An obsolete definition equated an electron dono ...
, while in the ferric state (Fe3+) it acts as an acceptor
Acceptor may refer to:
* Acceptor (accounting), the addressee of a bill of exchange
* In the Indian Contract Act of 1872, the acceptor is the person to whom a proposal is made, and who has communicated his or her acceptance of the said proposal
* ...
. Thus, iron plays a vital role in the catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
of enzymatic reactions that involve electron transfer (reduction and oxidation, redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
). Proteins can contain iron as part of different cofactors
Cofactor may also refer to:
* Cofactor (biochemistry), a substance that needs to be present in addition to an enzyme for a certain reaction to be catalysed
* A domain parameter in elliptic curve cryptography, defined as the ratio between the order ...
, such as iron–sulfur cluster
Iron–sulfur clusters are molecular ensembles of iron and sulfide. They are most often discussed in the context of the biological role for iron–sulfur proteins, which are pervasive. Many Fe–S clusters are known in the area of organometall ...
s (Fe-S) and heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
groups, both of which are assembled in mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
.
Cellular respiration
Human cells require iron in order to obtain energy as ATP from a multi-step process known as cellular respiration, more specifically fromoxidative phosphorylation
Oxidative phosphorylation(UK , US : or electron transport-linked phosphorylation or terminal oxidation, is the metabolic pathway in which Cell (biology), cells use enzymes to Redox, oxidize nutrients, thereby releasing chemical energy in order ...
at the mitochondrial cristae
A crista (; : cristae) is a fold in the inner membrane of a mitochondrion. The name is from the Latin for ''crest'' or ''plume'', and it gives the inner membrane its characteristic wrinkled shape, providing a large amount of surface area for che ...
. Iron is present in the iron–sulfur cluster and heme groups of the electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
proteins that generate a proton gradient
An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts:
* The chemical gradient, or difference in solute concentration across a membrane.
...
that allows ATP synthase
ATP synthase is an enzyme that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). ATP synthase is a molecular machine. The overall reaction catalyzed ...
to synthesize ATP (chemiosmosis
Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate, adenosine triphosphate (ATP) by the movement of hydrogen ion ...
).
Heme groups are part of hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
, a protein found in red blood cells that serves to transport oxygen from the lung
The lungs are the primary Organ (biology), organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the Vertebral column, backbone on either side of the heart. Their ...
s to other tissues. Heme groups are also present in myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle, skeletal Muscle, muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compar ...
to store and diffuse oxygen in muscle cells.
Oxygen transport
The human body needs iron for oxygen transport. Oxygen (O2) is required for the functioning and survival of nearly all cell types. Oxygen is transported from the lungs to the rest of the body bound to theheme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
group of hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
in red blood cells. In muscle cells, iron binds oxygen to myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle, skeletal Muscle, muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compar ...
, which regulates its release.
Toxicity
Iron is also potentially toxic. Its ability to donate and accept electrons means that it can catalyze the conversion ofhydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
into free radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired electron, unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemical reaction, chemi ...
. Free radicals can cause damage to a wide variety of cellular structures, and ultimately kill the cell.
Iron bound to proteins or cofactors such as heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
is safe. Also, there are virtually no truly free iron ions in the cell, since they readily form complexes with organic molecules. However, some of the intracellular iron is bound to low-affinity complexes, and is termed labile iron or "free" iron. Iron in such complexes can cause damage as described above.
To prevent that kind of damage, all life forms that use iron bind the iron atoms to proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, re ...
. This binding allows cells to benefit from iron while also limiting its ability to do harm. Typical intracellular labile iron concentrations in bacteria are 10-20 micromolar, though they can be 10-fold higher in anaerobic environment, where free radicals and reactive oxygen species
In chemistry and biology, reactive oxygen species (ROS) are highly Reactivity (chemistry), reactive chemicals formed from diatomic oxygen (), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (H2O2), superoxide (O2−), hydroxyl ...
are scarcer. In mammalian cells, intracellular labile iron concentrations are typically smaller than 1 micromolar, less than 5 percent of total cellular iron.
Bacterial protection
In response to a systemic bacterial infection, the immune system initiates a process known as " iron withholding". If bacteria are to survive, then they must obtain iron from their environment. Disease-causing bacteria do this in many ways, including releasing iron-binding molecules calledsiderophores
Siderophores (Greek: "iron carrier") are small, high-affinity iron-Chelation, chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore func ...
and then reabsorbing them to recover iron, or scavenging iron from hemoglobin and transferrin
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Iron(III), Fe3+ ions. Human transferrin is ...
. The harder the bacteria have to work to get iron, the greater a metabolic price they must pay. That means that iron-deprived bacteria reproduce more slowly. So, control of iron levels appears to be an important defense against many bacterial infections. Certain bacteria species have developed strategies to circumvent that defense, TB causing bacteria can reside within macrophages
Macrophages (; abbreviated MPhi, φ, MΦ or MP) are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris and foreign substances, which do not have proteins that ...
, which present an iron rich environment and ''Borrelia burgdorferi
''Borrelia burgdorferi'' is a bacterial species of the spirochete class in the genus '' Borrelia'', and is one of the causative agents of Lyme disease in humans. Along with a few similar genospecies, some of which also cause Lyme disease, it m ...
'' uses manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
in place of iron. People with increased amounts of iron, as, for example, in hemochromatosis, are more susceptible to some bacterial infections.
Although this mechanism is an elegant response to short-term bacterial infection, it can cause problems when it goes on so long that the body is deprived of needed iron for red cell production. Inflammatory cytokines
Cytokines () are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling.
Cytokines are produced by a broad range of cells, including immune cells like macrophages, B cell, B lymphocytes, T cell, T lymphocytes ...
stimulate the liver to produce the iron metabolism regulator protein hepcidin
Hepcidin is a protein that in humans is encoded by the ''HAMP'' gene. Hepcidin is a key regulator of the entry of iron into the circulation in mammals.
During conditions in which the hepcidin level is abnormally high, such as inflammation, se ...
, that reduces available iron. If hepcidin levels increase because of non-bacterial sources of inflammation, like viral infection, cancer, auto-immune diseases or other chronic diseases, then the anemia of chronic disease
Anemia of chronic disease (ACD) or anemia of chronic inflammation is a form of anemia seen in chronic infection, chronic immune activation, and malignancy. These conditions all produce elevation of interleukin-6, which stimulates hepcidin produc ...
may result. In this case, iron withholding actually impairs health by preventing the manufacture of enough hemoglobin-containing red blood cells.
Body iron stores
ferritin
Ferritin is a universal intracellular and extracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. ...
complexes that are present in all cells, but most common in bone marrow, liver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of var ...
, and spleen
The spleen (, from Ancient Greek '' σπλήν'', splḗn) is an organ (biology), organ found in almost all vertebrates. Similar in structure to a large lymph node, it acts primarily as a blood filter.
The spleen plays important roles in reg ...
. The liver stores of ferritin are the primary physiologic source of reserve iron in the body. The reserves of iron in industrialized countries tend to be lower in children and women of child-bearing age than in men and in the elderly. Women who must use their stores to compensate for iron lost through menstruation
Menstruation (also known as a period, among other colloquial terms) is the regular discharge of blood and Mucous membrane, mucosal tissue from the endometrium, inner lining of the uterus through the vagina. The menstrual cycle is characterized ...
, pregnancy
Pregnancy is the time during which one or more offspring gestation, gestates inside a woman's uterus. A multiple birth, multiple pregnancy involves more than one offspring, such as with twins.
Conception (biology), Conception usually occurs ...
or lactation
Lactation describes the secretion of milk from the mammary glands and the period of time that a mother lactates to feed her young. The process naturally occurs with all sexually mature female mammals, although it may predate mammals. The process ...
have lower non-hemoglobin body stores, which may consist of , or even less.
Of the body's total iron content, about is devoted to cellular proteins that use iron for important cellular processes like storing oxygen (myoglobin) or performing energy-producing redox reactions (cytochrome
Cytochromes are redox-active proteins containing a heme, with a central iron (Fe) atom at its core, as a cofactor. They are involved in the electron transport chain and redox catalysis. They are classified according to the type of heme and its ...
s). A relatively small amount (3–4 mg) circulates through the plasma, bound to transferrin. Because of its toxicity, free soluble iron is kept in low concentration in the body.
Iron deficiency
Iron deficiency, or sideropenia, is the state in which a body lacks enough iron to supply its needs. Iron is present in all cells in the human body and has several vital functions, such as carrying oxygen to the tissues from the lungs as a key ...
first affects the storage of iron in the body, and depletion of these stores is thought to be relatively asymptomatic, although some vague and non-specific symptom
Signs and symptoms are diagnostic indications of an illness, injury, or condition.
Signs are objective and externally observable; symptoms are a person's reported subjective experiences.
A sign for example may be a higher or lower temperature ...
s have been associated with it. Since iron is primarily required for hemoglobin, iron deficiency anemia
Iron-deficiency anemia is anemia caused by a lack of iron. Anemia is defined as a decrease in the number of red blood cells or the amount of hemoglobin in the blood. When onset is slow, symptoms are often vague such as feeling tired, weak, sh ...
is the primary clinical manifestation of iron deficiency. Iron-deficient people will suffer or die from organ damage well before their cells run out of the iron needed for intracellular processes like electron transport.
Macrophages
Macrophages (; abbreviated MPhi, φ, MΦ or MP) are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris and foreign substances, which do not have proteins that ...
of the reticuloendothelial system
In anatomy the term reticuloendothelial system (abbreviated RES), often associated nowadays with the mononuclear phagocyte system (MPS), was employed by the beginning of the 20th century to denote a system of specialised cells that effectively cl ...
store iron as part of the process of breaking down and processing hemoglobin from engulfed red blood cells. Iron is also stored as a pigment called hemosiderin
Hemosiderin image of a kidney viewed under a microscope. The brown areas represent hemosiderin
Hemosiderin or haemosiderin is an iron-storage complex that is composed of partially digested ferritin and lysosomes. The breakdown of heme gives ri ...
, which is an ill-defined deposit of protein and iron, created by macrophages where excess iron is present, either locally or systemically, e.g., among people with iron overload due to frequent blood cell destruction and the necessary transfusions their condition calls for. If systemic iron overload is corrected, over time the hemosiderin is slowly resorbed by the macrophages.
Mechanisms of iron regulation
 Human iron homeostasis is regulated at two different levels. Systemic iron levels are balanced by the controlled absorption of dietary iron by
Human iron homeostasis is regulated at two different levels. Systemic iron levels are balanced by the controlled absorption of dietary iron by enterocytes
Enterocytes, or intestinal absorptive cells, are simple columnar epithelial cells which line the inner surface of the small and large intestines. A glycocalyx surface coat contains digestive enzymes. Microvilli on the apical surface increase i ...
, the cells that line the interior of the intestines
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The tract is the largest of the body's systems, after the cardiovascular system. ...
, and the uncontrolled loss of iron from epithelial sloughing, sweat, injuries and blood loss. In addition, systemic iron is continuously recycled. Cellular iron levels are controlled differently by different cell types due to the expression of particular iron regulatory and transport proteins.
Systemic iron regulation

Dietary iron uptake
The absorption of dietary iron is a variable and dynamic process. The amount of iron absorbed compared to the amount ingested is typically low, but may range from 5% to as much as 35% depending on circumstances and type of iron. The efficiency with which iron is absorbed varies depending on the source. Generally, the best-absorbed forms of iron come from animal products. Absorption of dietary iron in iron salt form (as in most supplements) varies somewhat according to the body's need for iron, and is usually between 10% and 20% of iron intake. Absorption of iron from animal products, and some plant products, is in the form of heme iron, and is more efficient, allowing absorption of from 15% to 35% of intake. Heme iron in animals is from blood and heme-containing proteins in meat and mitochondria, whereas in plants, heme iron is present in mitochondria in all cells that use oxygen for respiration. Like most mineral nutrients, the majority of the iron absorbed from digested food or supplements is absorbed in theduodenum
The duodenum is the first section of the small intestine in most vertebrates, including mammals, reptiles, and birds. In mammals, it may be the principal site for iron absorption.
The duodenum precedes the jejunum and ileum and is the shortest p ...
by enterocyte
Enterocytes, or intestinal absorptive cells, are simple columnar epithelial cells which line the inner surface of the small and large intestines. A glycocalyx surface coat contains digestive enzymes. Microvilli on the apical surface increase ...
s of the duodenal lining. These cells have special molecules that allow them to move iron into the body. To be absorbed, dietary iron can be absorbed as part of a protein such as heme protein or iron must be in its ferrous Fe2+ form. A ferric reductase enzyme on the enterocytes' brush border
A brush border (striated border or brush border membrane) is the microvillus-covered surface of simple cuboidal and simple columnar epithelium found in different parts of the body. Microvilli are approximately 100 nanometers in diameter and th ...
, duodenal cytochrome B ( Dcytb), reduces ferric Fe3+ to Fe2+. A protein called divalent metal transporter 1 (DMT1
Natural resistance-associated macrophage protein 2 (NRAMP 2), also known as divalent metal transporter 1 (DMT1) and divalent cation transporter 1 (DCT1), is a protein that in humans is encoded by the ''SLC11A2'' (solute carrier family 11, member 2 ...
), which can transport several divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemica ...
metals across the plasma membrane, then transports iron across the enterocyte's cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
into the cell. If the iron is bound to heme, it is instead transported across the apical membrane by heme carrier protein 1 (HCP1). Heme is then catabolized
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipid ...
by microsomal heme oxygenase
Heme oxygenase, or haem oxygenase, (HMOX, commonly abbreviated as HO) is an enzyme that catalyzes the degradation of heme to produce biliverdin, ferrous iron, and carbon monoxide.
There are many heme degrading enzymes in nature. In general, on ...
into biliverdin
Biliverdin (from the Latin for green bile) is a green tetrapyrrolic bile pigment, and is a product of heme catabolism.Boron W, Boulpaep E. Medical Physiology: a cellular and molecular approach, 2005. 984–986. Elsevier Saunders, United States. ...
, releasing Fe2+.
These intestinal lining cells can then either store the iron as ferritin
Ferritin is a universal intracellular and extracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. ...
, which is accomplished by Fe2+ binding to apoferritin (in which case the iron will leave the body when the cell dies and is sloughed off into feces
Feces (also known as faeces American and British English spelling differences#ae and oe, or fæces; : faex) are the solid or semi-solid remains of food that was not digested in the small intestine, and has been broken down by bacteria in the ...
), or the cell can release it into the body via the only known iron exporter in mammals, ferroportin
Ferroportin-1, also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1), is a protein that in humans is encoded by the ''SLC40A1'' gene. Ferroportin is a transmembrane protein that transports iron from the ...
. Hephaestin
Hephaestin, also known as HEPH, is a protein which in humans is encoded by the ''HEPH'' gene.
Function
Hephaestin is involved in the metabolism and homeostasis of iron and possibly copper. It is a transmembrane copper-dependent ferroxidase resp ...
, a ferroxidase
Ferroxidase also known as Fe(II):oxygen oxidoreductase is an enzyme that catalyzes the oxidization of iron II to iron III:
: 4 Fe2+ + 4 H+ + O2 ⇔ 4 Fe3+ + 2H2O
Examples
Human genes encoding proteins with ferroxidase activity include:
* C ...
that can oxidize Fe2+ to Fe3+ and is found mainly in the small intestine, helps ferroportin transfer iron across the basolateral end of the intestine cells. Upon release into the bloodstream, Fe3+ binds transferrin and circulates to tissues. In contrast, ferroportin is post-translationally repressed by hepcidin
Hepcidin is a protein that in humans is encoded by the ''HAMP'' gene. Hepcidin is a key regulator of the entry of iron into the circulation in mammals.
During conditions in which the hepcidin level is abnormally high, such as inflammation, se ...
, a 25-amino acid peptide hormone. The body regulates iron levels by regulating each of these steps. For instance, enterocytes synthesize more Dcytb, DMT1 and ferroportin in response to iron deficiency anemia. Iron absorption from diet is enhanced in the presence of vitamin C and diminished by excess calcium, zinc, or manganese.
The human body's rate of iron absorption appears to respond to a variety of interdependent factors, including total iron stores, the extent to which the bone marrow is producing new red blood cells, the concentration of hemoglobin in the blood, and the oxygen content of the blood. The body also absorbs less iron during times of inflammation
Inflammation (from ) is part of the biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants. The five cardinal signs are heat, pain, redness, swelling, and loss of function (Latin ''calor'', '' ...
, in order to deprive bacteria of iron. Recent discoveries demonstrate that hepcidin regulation of ferroportin is responsible for the syndrome of anemia of chronic disease.
Iron recycling and loss
Most of the iron in the body is hoarded and recycled by the reticuloendothelial system, which breaks down aged red blood cells. In contrast to iron uptake and recycling, there is no physiologic regulatory mechanism for excreting iron. People lose a small but steady amount by gastrointestinal blood loss, sweating and by shedding cells of the skin and themucosa
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It ...
l lining of the gastrointestinal tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the Digestion, digestive system that leads from the mouth to the anus. The tract is the largest of the body's systems, after the cardiovascula ...
. The total amount of loss for healthy people in the developed world amounts to an estimated average of a day for men, and 1.5–2 mg a day for women with regular menstrual periods. People with gastrointestinal parasitic infections, more commonly found in developing countries, often lose more. Those who cannot regulate absorption well enough get disorders of iron overload. In these diseases, the toxicity of iron starts overwhelming the body's ability to bind and store it.
Cellular iron regulation
Iron import
Most cell types take up iron primarily throughreceptor-mediated endocytosis
Receptor-mediated endocytosis (RME), also called clathrin-mediated endocytosis, is a process by which cells absorb metabolites, hormones, proteins – and in some cases viruses – by the inward budding of the plasma membrane (invagination). This ...
via transferrin receptor 1
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded b ...
(TFR1), transferrin receptor 2 (TFR2) and GAPDH
Glyceraldehyde 3-phosphate dehydrogenase (abbreviated GAPDH) () is an enzyme of about 37kDa that catalyzes the sixth step of glycolysis and thus serves to break down glucose for energy and carbon molecules. In addition to this long establish ...
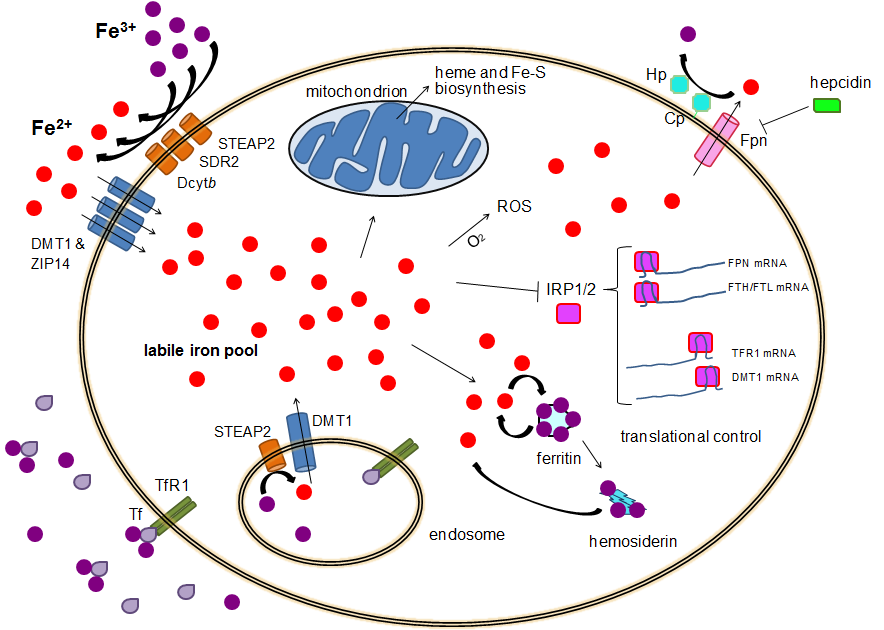
. TFR1 has a 30-fold higher affinity for transferrin-bound iron than TFR2 and thus is the main player in this process. The higher order multifunctional glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) also acts as a transferrin receptor. Transferrin-bound ferric iron is recognized by these transferrin receptors, triggering a conformational change that causes endocytosis. Iron then enters the cytoplasm from the endosome via importer DMT1 after being reduced to its ferrous state by a STEAP family reductase.
Alternatively, iron can enter the cell directly via plasma membrane divalent cation importers such as DMT1 and ZIP14 (Zrt-Irt-like protein 14). Again, iron enters the cytoplasm in the ferrous state after being reduced in the extracellular space by a reductase such as STEAP2, STEAP3 (in red blood cells), Dcytb (in enterocytes) and SDR2.
Iron import in some cancer cells
Iron can also enter cells viaCD44
The CD44 antigen is a cell-surface glycoprotein involved in cell–cell interactions, cell adhesion and migration. In humans, the CD44 antigen is encoded by the ''CD44'' gene on chromosome 11. CD44 has been referred to as HCAM (homing cell adhes ...
in complexes bound to hyaluronic acid
Hyaluronic acid (; abbreviated HA; conjugate base hyaluronate), also called hyaluronan, is an anionic, nonsulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. It is unique among glycosaminog ...
during epithelial–mesenchymal transition
The epithelial–mesenchymal transition (EMT) is a process by which epithelial cells lose their cell polarity and cell–cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells; these are multipotent stromal ...
(EMT). In this process, epithelial cells
Epithelium or epithelial tissue is a thin, continuous, protective layer of cells with little extracellular matrix. An example is the epidermis, the outermost layer of the skin. Epithelial ( mesothelial) tissues line the outer surfaces of man ...
transform into mesenchymal cells
Mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells or medicinal signaling cells, are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts (bone cells), chondrocytes (cartilage c ...
with detachment from the basement membrane
The basement membrane, also known as base membrane, is a thin, pliable sheet-like type of extracellular matrix that provides cell and tissue support and acts as a platform for complex signalling. The basement membrane sits between epithelial tis ...
, to which they’re normally anchored, paving the way for the newly differentiated motile mesenchymal cells to begin migration away from the epithelial layer.
While EMT plays a crucial role in physiological processes like implantation, where it enables the embryo
An embryo ( ) is the initial stage of development for a multicellular organism. In organisms that reproduce sexually, embryonic development is the part of the life cycle that begins just after fertilization of the female egg cell by the male sp ...
to invade the endometrium
The endometrium is the inner epithelium, epithelial layer, along with its mucous membrane, of the mammalian uterus. It has a basal layer and a functional layer: the basal layer contains stem cells which regenerate the functional layer. The funct ...
to facilitate placental
Placental mammals (infraclass Placentalia ) are one of the three extant subdivisions of the class Mammalia, the other two being Monotremata and Marsupialia. Placentalia contains the vast majority of extant mammals, which are partly distinguished ...
attachment, its dysregulation can also fuel the malignant
Malignancy () is the tendency of a medical condition to become progressively worse; the term is most familiar as a characterization of cancer.
A ''malignant'' tumor contrasts with a non-cancerous benign tumor, ''benign'' tumor in that a malig ...
spread of tumors
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
empowering them to invade surrounding tissues and establish distant colonies (metastasis
Metastasis is a pathogenic agent's spreading from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, ...
).
Malignant cells often exhibit a heightened demand for iron, fueling their transition towards a more invasive mesenchymal state. This iron is necessary for the expression of mesenchymal genes, like those encoding transforming growth factor beta
Transforming growth factor beta (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms (TGF-β 1 to 3, HGNC symbols TGFB1, TGFB2, TGFB3) and many other ...
(TGF-β), crucial for EMT. Notably, iron’s unique ability to catalyze protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
and DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
demethylation
Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen at ...
plays a vital role in this gene expression process.
Conventional iron uptake pathways, such as those using the transferrin receptor 1 (TfR1), often prove insufficient to meet these elevated iron demands in cancer cells. As a result, various cytokines
Cytokines () are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling.
Cytokines are produced by a broad range of cells, including immune cells like macrophages, B cell, B lymphocytes, T cell, T lymphocytes ...
and growth factors
A growth factor is a naturally occurring substance capable of stimulating cell proliferation, wound healing, and occasionally cellular differentiation. Usually it is a secreted protein or a steroid hormone. Growth factors are important for regu ...
trigger the upregulation of CD44, a surface molecule capable of internalizing iron bound to the hyaluronan complex. This alternative pathway, relying on CD44-mediated endocytosis, becomes the dominant iron uptake mechanism compared to the traditional TfR1-dependent route.
The labile iron pool
In the cytoplasm, ferrous iron is found in a soluble, chelatable state which constitutes the labile iron pool (~0.001 mM). In this pool, iron is thought to be bound to low-mass compounds such as peptides, carboxylates and phosphates, although some might be in a free, hydrated form ( aqua ions). Alternatively, iron ions might be bound to specialized proteins known as metallochaperones. Specifically, poly-r(C)-binding proteins PCBP1 andPCBP2
Poly(rC)-binding protein 2 is a protein that in humans is encoded by the ''PCBP2'' gene.
Function
The protein encoded by this gene appears to be multifunctional. It along with PCBP-1 and hnRNPK corresponds to the major cellular poly(rC)-bindi ...
appear to mediate transfer of free iron to ferritin (for storage) and non-heme iron enzymes (for use in catalysis). The labile iron pool is potentially toxic due to iron's ability to generate reactive oxygen species. Iron from this pool can be taken up by mitochondria via mitoferrin to synthesize Fe-S clusters and heme groups.
The storage iron pool
Iron can be stored in ferritin as ferric iron due to theferroxidase
Ferroxidase also known as Fe(II):oxygen oxidoreductase is an enzyme that catalyzes the oxidization of iron II to iron III:
: 4 Fe2+ + 4 H+ + O2 ⇔ 4 Fe3+ + 2H2O
Examples
Human genes encoding proteins with ferroxidase activity include:
* C ...
activity of the ferritin heavy chain. Dysfunctional ferritin may accumulate as hemosiderin
Hemosiderin image of a kidney viewed under a microscope. The brown areas represent hemosiderin
Hemosiderin or haemosiderin is an iron-storage complex that is composed of partially digested ferritin and lysosomes. The breakdown of heme gives ri ...
, which can be problematic in cases of iron overload. The ferritin storage iron pool is much larger than the labile iron pool, ranging in concentration from 0.7 mM to 3.6 mM.
Iron export
Iron export occurs in a variety of cell types, includingneuron
A neuron (American English), neurone (British English), or nerve cell, is an membrane potential#Cell excitability, excitable cell (biology), cell that fires electric signals called action potentials across a neural network (biology), neural net ...
s, red blood cells, hepatocytes, macrophages and enterocytes. The latter two are especially important since systemic iron levels depend upon them. There is only one known iron exporter, ferroportin
Ferroportin-1, also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1), is a protein that in humans is encoded by the ''SLC40A1'' gene. Ferroportin is a transmembrane protein that transports iron from the ...
. It transports ferrous iron out of the cell, generally aided by ceruloplasmin
Ceruloplasmin (or caeruloplasmin) is a ferroxidase enzyme that in humans is encoded by the ''CP'' gene.
Ceruloplasmin is the major copper-carrying protein in the blood, and in addition plays a role in iron metabolism. It was first described in ...
and/or hephaestin
Hephaestin, also known as HEPH, is a protein which in humans is encoded by the ''HEPH'' gene.
Function
Hephaestin is involved in the metabolism and homeostasis of iron and possibly copper. It is a transmembrane copper-dependent ferroxidase resp ...
(mostly in enterocytes), which oxidize iron to its ferric state so it can bind ferritin in the extracellular medium. Hepcidin
Hepcidin is a protein that in humans is encoded by the ''HAMP'' gene. Hepcidin is a key regulator of the entry of iron into the circulation in mammals.
During conditions in which the hepcidin level is abnormally high, such as inflammation, se ...
causes the internalization of ferroportin, decreasing iron export. Besides, hepcidin seems to downregulate both TFR1 and DMT1 through an unknown mechanism. Another player assisting ferroportin in effecting cellular iron export is GAPDH. A specific post translationally modified isoform of GAPDH is recruited to the surface of iron loaded cells where it recruits apo-transferrin in close proximity to ferroportin so as to rapidly chelate the iron extruded.
 The expression of hepcidin, which only occurs in certain cell types such as
The expression of hepcidin, which only occurs in certain cell types such as hepatocyte
A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass.
These cells are involved in:
* Protein synthesis
* Protein storage
* Transformation of carbohydrates
* Synthesis of cholesterol, bi ...
s, is tightly controlled at the transcriptional level and it represents the link between cellular and systemic iron homeostasis due to hepcidin's role as "gatekeeper" of iron release from enterocytes into the rest of the body. Erythroblasts
A nucleated red blood cell (NRBC), also known by several other names, is a red blood cell that contains a cell nucleus. Almost all vertebrate organisms have hemoglobin-containing cells in their blood, and with the exception of mammals, all of th ...
produce erythroferrone
Erythroferrone is a protein hormone encoded in humans by the ''ERFE'' gene. Erythroferrone is produced by erythroblasts, inhibits the production of hepcidin in the liver, and so increases the amount of iron metabolism, iron available for hemogl ...
, a hormone which inhibits hepcidin and so increases the availability of iron needed for hemoglobin synthesis.
Translational control of cellular iron
Although some control exists at the transcriptional level, the regulation of cellular iron levels is ultimately controlled at the translational level byiron-responsive element-binding protein
The iron-responsive element-binding proteins, also known as IRE-BP, IRBP, IRP and IFR
, bind to iron-responsive elements (IREs) in the regulation of human iron metabolism.
Function
ACO1, or IRP1, is a bifunctional protein that functions as an ...
s IRP1 and especially IRP2. When iron levels are low, these proteins are able to bind to iron-responsive elements (IREs). IREs are stem loop structures in the untranslated regions (UTRs) of mRNA.
Both ferritin and ferroportin contain an IRE in their 5' UTRs, so that under iron deficiency their translation is repressed by IRP2, preventing the unnecessary synthesis of storage protein and the detrimental export of iron. In contrast, TFR1 and some DMT1 variants contain 3' UTR IREs, which bind IRP2 under iron deficiency, stabilizing the mRNA, which guarantees the synthesis of iron importers.
Pathology
Iron deficiency
phytates
Phytic acid is a six-fold dihydrogenphosphate ester of inositol (specifically, of the ''myo'' isomer), also called inositol hexaphosphate, inositol hexakisphosphate (IP6) or inositol polyphosphate. At physiological pH, the phosphates are partial ...
in bran
Bran, also known as miller's bran, is the component of a Cereal, cereal grain consisting of the hard layersthe combined aleurone and Fruit anatomy#Pericarp layers, pericarpsurrounding the endosperm. Maize, Corn (maize) bran also includes the p ...
, calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
from supplements or dairy products, and tannins
Tannins (or tannoids) are a class of astringent, polyphenolic biomolecules that bind to and precipitate proteins and various other organic compounds including amino acids and alkaloids. The term ''tannin'' is widely applied to any large po ...
from tea, although in all three of these studies the effect was small and the authors of the studies cited regarding bran and tea note that the effect will probably only have a noticeable impact when most iron is obtained from vegetable sources.
* Acid-reducing medications: Acid-reducing medications reduce the absorption of dietary iron. These medications are commonly used for gastritis, reflux disease, and ulcers. Proton pump inhibitors (PPIs), H2 antihistamines, and antacids will reduce iron metabolism.
* Damage to the intestinal lining. Examples of causes of this kind of damage include surgery involving the duodenum or diseases like Crohn's
Crohn's disease is a type of inflammatory bowel disease (IBD) that may affect any segment of the gastrointestinal tract. Symptoms often include abdominal pain, diarrhea, fever, abdominal distension, and weight loss. Complications outside of the ...
or celiac sprue
Coeliac disease (British English) or celiac disease (American English) is a long-term autoimmune disorder, primarily affecting the small intestine. Patients develop hypersensitivity, intolerance to gluten, which is present in foods such as wh ...
which severely reduce the surface area available for absorption. ''Helicobacter pylori
''Helicobacter pylori'', previously known as ''Campylobacter pylori'', is a gram-negative, Flagellum#bacterial, flagellated, Bacterial cellular morphologies#Helical, helical bacterium. Mutants can have a rod or curved rod shape that exhibits l ...
'' infections also reduce the availability of iron.
* Inflammation leading to hepcidin-induced restriction on iron release from enterocytes (see above).
* Is also a common occurrence in pregnant women, and in growing adolescents due to poor diets.
* Acute blood loss or acute liver cirrhosis creates a lack of transferrin therefore causing iron to be secreted from the body.
Iron overload
The body is able to substantially reduce the amount of iron it absorbs across the mucosa. It does not seem to be able to entirely shut down the iron transport process. Also, in situations where excess iron damages the intestinal lining itself (for instance, when children eat a large quantity of iron tablets produced for adult consumption), even more iron can enter the bloodstream and cause a potentially deadly syndrome of iron overload. Large amounts of free iron in the circulation will cause damage to critical cells in the liver, theheart
The heart is a muscular Organ (biology), organ found in humans and other animals. This organ pumps blood through the blood vessels. The heart and blood vessels together make the circulatory system. The pumped blood carries oxygen and nutrie ...
and other metabolically active organs.
Iron toxicity results when the amount of circulating iron exceeds the amount of transferrin available to bind it, but the body is able to vigorously regulate its iron uptake. Thus, iron toxicity from ingestion is usually the result of extraordinary circumstances like iron tablet over-consumption rather than variations in diet
Diet may refer to:
Food
* Diet (nutrition), the sum of the food consumed by an organism or group
* Dieting, the deliberate selection of food to control body weight or nutrient intake
** Diet food, foods that aid in creating a diet for weight loss ...
. The type of acute toxicity from iron ingestion causes severe mucosal damage in the gastrointestinal tract, among other problems.
Excess iron has been linked to higher rates of disease and mortality. For example, breast cancer patients with low ferroportin
Ferroportin-1, also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1), is a protein that in humans is encoded by the ''SLC40A1'' gene. Ferroportin is a transmembrane protein that transports iron from the ...
expression (leading to higher concentrations of intracellular iron) survive for a shorter period of time on average, while high ferroportin expression predicts 90% 10-year survival in breast cancer patients. Similarly, genetic variations in iron transporter genes known to increase serum iron levels also reduce lifespan and the average number of years spent in good health. It has been suggested that mutations that increase iron absorption, such as the ones responsible for hemochromatosis (see below), were selected for during Neolithic
The Neolithic or New Stone Age (from Ancient Greek, Greek 'new' and 'stone') is an archaeological period, the final division of the Stone Age in Mesopotamia, Asia, Europe and Africa (c. 10,000 BCE to c. 2,000 BCE). It saw the Neolithic Revo ...
times as they provided a selective advantage
In biology, adaptation has three related meanings. Firstly, it is the dynamic evolutionary process of natural selection that fits organisms to their environment, enhancing their evolutionary fitness. Secondly, it is a state reached by the po ...
against iron-deficiency anemia. The increase in systemic iron levels becomes pathological in old age, which supports the notion that antagonistic pleiotropy or "hyperfunction" drives human aging.
Chronic iron toxicity is usually the result of more chronic iron overload syndromes associated with genetic diseases, repeated transfusions or other causes. In such cases the iron stores of an adult may reach 50 grams (10 times normal total body iron) or more. The most common diseases of iron overload are hereditary hemochromatosis
Hereditary haemochromatosis type 1 (HFE-related haemochromatosis) is a genetic disorder characterized by excessive intestinal absorption of Human iron metabolism, dietary iron, resulting in a pathological increase in total body iron stores. Huma ...
(HH), caused by mutations in the '' HFE'' gene, and the more severe disease juvenile hemochromatosis
Juvenile hemochromatosis, also known as hemochromatosis type 2, is a rare form of hereditary hemochromatosis, which emerges in young individuals, typically between 15 and 30 years of age, but occasionally later. It is characterized by an inability ...
(JH), caused by mutations in either hemojuvelin
Hemojuvelin (HJV), also known as repulsive guidance molecule C (RGMc) or hemochromatosis type 2 protein (HFE2), is a membrane-bound and soluble protein in mammals that is responsible for the iron overload condition known as juvenile hemochromatos ...
(''HJV'') or hepcidin (''HAMP''). The exact mechanisms of most of the various forms of adult hemochromatosis, which make up most of the genetic iron overload disorders, remain unsolved. So, while researchers have been able to identify genetic mutations causing several adult variants of hemochromatosis, they now must turn their attention to the normal function of these mutated genes.
See also
* Iron in biologyReferences
Further reading
* electronic-book electronic- * * * * See esp. pp. 513-514. * * *External links
A comprehensive NIH factsheet on iron and nutrition
Iron Disorders Institute: A nonprofit group concerned with iron disorders; site has helpful links and information on iron-related medical disorders.
An interactive medical learning portal on iron metabolism
Information about iron outside the body
{{Authority control Hematology Human homeostasis Biology and pharmacology of chemical elements