Ionized Air Glow on:
[Wikipedia]
[Google]
[Amazon]

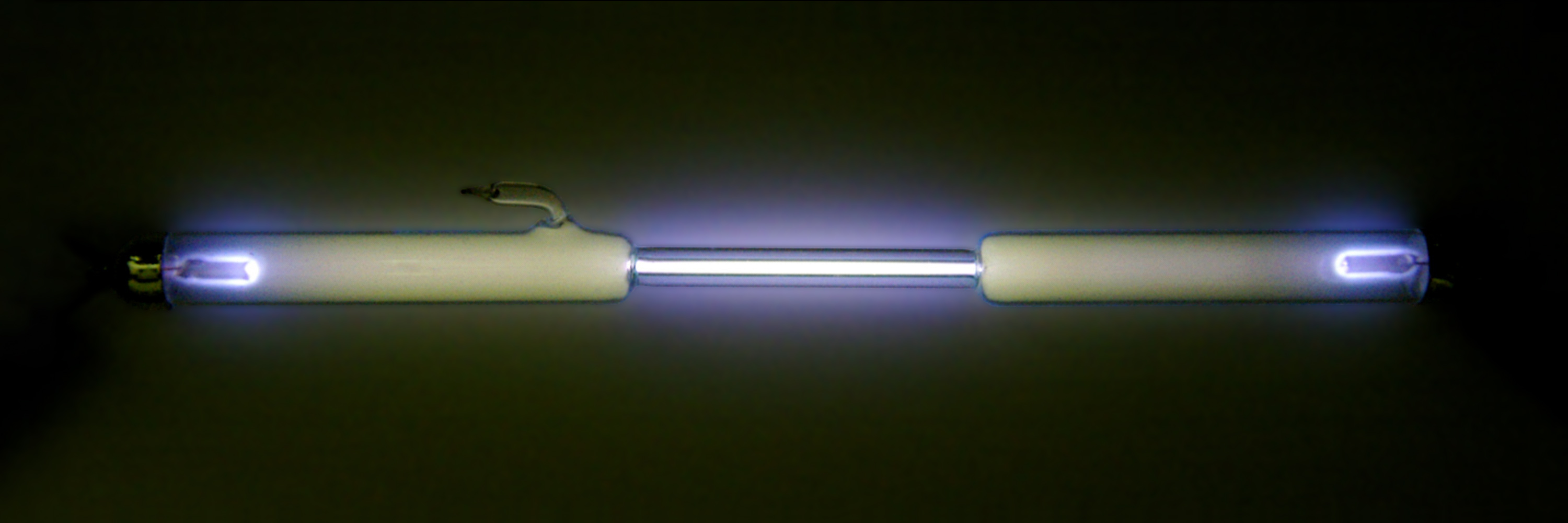

 Ionized-air glow is the
Ionized-air glow is the
 * Within minutes after the steam explosion that caused the
* Within minutes after the steam explosion that caused the


 In dry air, the color of produced light (e.g. by lightning) is dominated by the emission lines of nitrogen, yielding the spectrum with primarily blue emission lines. The lines of neutral nitrogen (NI), neutral oxygen (OI), singly ionized nitrogen (NII) and singly ionized oxygen (OII) are the most prominent features of a lightning
In dry air, the color of produced light (e.g. by lightning) is dominated by the emission lines of nitrogen, yielding the spectrum with primarily blue emission lines. The lines of neutral nitrogen (NI), neutral oxygen (OI), singly ionized nitrogen (NII) and singly ionized oxygen (OII) are the most prominent features of a lightning



 Ionized-air glow is the
Ionized-air glow is the luminescent
Luminescence is a spontaneous emission of radiation from an electronically or vibrationally excited species not in thermal equilibrium with its environment. A luminescent object emits ''cold light'' in contrast to incandescence, where an objec ...
emission of characteristic blue–purple–violet light, often of a color called electric blue, by air
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
subjected to an energy flux either directly or indirectly from solar radiation
Sunlight is the portion of the electromagnetic radiation which is emitted by the Sun (i.e. solar radiation) and received by the Earth, in particular the visible light perceptible to the human eye as well as invisible infrared (typically p ...
.
Processes
When energy is deposited in air, the air molecules become excited. As air is composed primarily ofnitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, excited N2 and O2 molecules are produced. These can react with other molecules, forming mainly ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
and nitrogen(II) oxide. Water vapor
Water vapor, water vapour, or aqueous vapor is the gaseous phase of Properties of water, water. It is one Phase (matter), state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from th ...
, when present, may also play a role; its presence is characterized by the hydrogen emission lines. The reactive species present in the plasma can readily react with other chemicals present in the air or on nearby surfaces.
Deexcitation of nitrogen
The excited nitrogen deexcites primarily by emission of aphoton
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
, with emission lines in ultraviolet, visible, and infrared band:
:N2* → N2 + ''hν''
The blue light observed is produced primarily by this process. The spectrum is dominated by lines of single-ionized nitrogen, with presence of neutral nitrogen lines.
Deexcitation of oxygen
Theexcited state
In quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Add ...
of oxygen is somewhat more stable than nitrogen. While deexcitation can occur by emission of photons, the more probable mechanism at atmospheric pressure is a chemical reaction with other oxygen molecules, forming ozone:
: O2* + 2 O2 → 2 O3
This reaction is responsible for the production of ozone in the vicinity of strongly radioactive materials and electrical discharges.
Occurrence
Excitation energy can be deposited in air by a number of different mechanisms: *Ionizing radiation
Ionizing (ionising) radiation, including Radioactive decay, nuclear radiation, consists of subatomic particles or electromagnetic waves that have enough energy per individual photon or particle to ionization, ionize atoms or molecules by detaching ...
is the cause of blue glow surrounding sufficient quantities of strongly radioactive materials in air, e.g. some radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
specimens (e.g. radium
Radium is a chemical element; it has chemical symbol, symbol Ra and atomic number 88. It is the sixth element in alkaline earth metal, group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, ...
or polonium
Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal (although sometimes classified as a metalloid) with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tel ...
), particle beam
A particle beam is a stream of charged particle, charged or neutral particles other than photons. In Particle accelerator, particle accelerators, these particles can move with a velocity close to the speed of light. There is a difference between ...
s (e.g. from particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel electric charge, charged particles to very high speeds and energies to contain them in well-defined particle beam, beams. Small accelerators are used for fundamental ...
s) in air, the blue flashes during criticality accident
A criticality accident is an accidental uncontrolled nuclear fission chain reaction. It is sometimes referred to as a critical excursion, critical power excursion, divergent chain reaction, or simply critical. Any such event involves the uninten ...
s, and the eerie/low brightness "purple" to "blue" glow enveloping a mushroom cloud
A mushroom cloud is a distinctive mushroom-shaped flammagenitus cloud of debris, smoke, and usually condensed water vapour resulting from a large explosion. The effect is most commonly associated with a nuclear explosion, but any sufficiently e ...
during the first several dozen seconds after nuclear explosion
A nuclear explosion is an explosion that occurs as a result of the rapid release of energy from a high-speed nuclear reaction. The driving reaction may be nuclear fission or nuclear fusion or a multi-stage cascading combination of the two, th ...
s near sea level. This post-explosion effect has been observed only at night from atmospheric nuclear tests owing to its low brightness, with observers noticing it following the pre-dawn Trinity test
Trinity was the first detonation of a nuclear weapon, conducted by the United States Army at 5:29 a.m. MWT (11:29:21 GMT) on July 16, 1945, as part of the Manhattan Project. The test was of an implosion-design plutonium bomb, or "gadg ...
, as well as Upshot-Knothole Annie, Operation Fishbowl, and the ''Cherokee'' shot of Operation Redwing
Operation Redwing was a United States series of 17 nuclear test detonations from May to July 1956. They were conducted at Bikini and Enewetak atolls by Joint Task Force 7 (JTF7).Blumenson, Martin and Hugh D. Hexamer (1956). ''A History of ...
.Cherokee Field Report Bikini Operations, page 10, quoted in
 * Within minutes after the steam explosion that caused the
* Within minutes after the steam explosion that caused the Chernobyl accident
On 26 April 1986, the no. 4 reactor of the Chernobyl Nuclear Power Plant, located near Pripyat, Ukrainian Soviet Socialist Republic, Ukrainian SSR, Soviet Union (now Ukraine), exploded. With dozens of direct casualties, it is one of only ...
at 01:23 local time, employees at the power station went outside to get a clearer view of the extent of the damage. One such survivor, Alexander Yuvchenko, recounts that once he stopped outside and looked up towards the reactor hall he saw a "very beautiful" laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
-like beam of light bluish light, caused by the ionization of air, that appeared to be "flooding up into infinity".
* Cathode ray
Cathode rays are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the c ...
s in air produce this blue glow.
* Electrical discharge in air is the cause of blue light emitted by electric spark
An electric spark is an abrupt electrical discharge that occurs when a sufficiently high electric field creates an Ionization, ionized, Electric current, electrically conductive channel through a normally-insulating medium, often air or other ga ...
s, lightning
Lightning is a natural phenomenon consisting of electrostatic discharges occurring through the atmosphere between two electrically charged regions. One or both regions are within the atmosphere, with the second region sometimes occurring on ...
, and corona discharge
A corona discharge is an electrical discharge caused by the ionization of a fluid such as air surrounding a conductor (material), conductor carrying a high voltage. It represents a local region where the air (or other fluid) has undergone ...
s (e.g. St. Elmo's fire).
* Auroras, the sometimes observable blue-violet hues emitted by nitrogen at lower altitudes.
Colors


 In dry air, the color of produced light (e.g. by lightning) is dominated by the emission lines of nitrogen, yielding the spectrum with primarily blue emission lines. The lines of neutral nitrogen (NI), neutral oxygen (OI), singly ionized nitrogen (NII) and singly ionized oxygen (OII) are the most prominent features of a lightning
In dry air, the color of produced light (e.g. by lightning) is dominated by the emission lines of nitrogen, yielding the spectrum with primarily blue emission lines. The lines of neutral nitrogen (NI), neutral oxygen (OI), singly ionized nitrogen (NII) and singly ionized oxygen (OII) are the most prominent features of a lightning emission spectrum
The emission spectrum of a chemical element or chemical compound is the Spectrum (physical sciences), spectrum of frequencies of electromagnetic radiation emitted due to electrons making a atomic electron transition, transition from a high energ ...
. Neutral nitrogen radiates primarily at one line in the red part of the spectrum. Ionized nitrogen radiates primarily as a set of lines in the blue part of the spectrum.
A violet hue can occur when the spectrum contains emission lines of atomic hydrogen. This may happen when the air contains high amount of water, e.g. with lightnings in low altitudes passing through rain
Rain is a form of precipitation where water drop (liquid), droplets that have condensation, condensed from Water vapor#In Earth's atmosphere, atmospheric water vapor fall under gravity. Rain is a major component of the water cycle and is res ...
thunderstorm
A thunderstorm, also known as an electrical storm or a lightning storm, is a storm characterized by the presence of lightning and its acoustics, acoustic effect on the Earth's atmosphere, known as thunder. Relatively weak thunderstorm ...
s. Water vapor and small water droplets ionize and dissociate easier than large droplets, therefore have higher impact on color.
The hydrogen emission lines at 656.3 nm (the strong H-alpha
Hydrogen-alpha, typically shortened to H-alpha or Hα, is a deep-red visible spectral line of the hydrogen atom with a wavelength of 656.28 nm in air and 656.46 nm in vacuum. It is the first spectral line in the Balmer series and is em ...
line) and at 486.1 nm (H-beta) are characteristic for lightnings.
Rydberg atom
A Rydberg atom is an excited atom with one or more electrons that have a very high principal quantum number, ''n''. The higher the value of ''n'', the farther the electron is from the nucleus, on average. Rydberg atoms have a number of peculi ...
s, generated by low-frequency lightnings, emit at red to orange color and can give the lightning a yellowish to greenish tint.( confusing?)
Generally, the radiant species present in atmospheric plasma are N2, N2+, O2, NO (in dry air) and OH (in humid air). The temperature, electron density
Electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typical ...
, and electron temperature of the plasma can be inferred from the distribution of rotational lines of these species. At higher temperatures, atomic emission lines of N and O, and (in presence of water) H, are present. Other molecular lines, e.g. CO and CN, mark the presence of contaminants in the air.
Cherenkov radiation
The emission of blue light is often attributed toCherenkov radiation
Cherenkov radiation () is electromagnetic radiation emitted when a charged particle (such as an electron) passes through a dielectric medium (such as distilled water) at a speed greater than the phase velocity (speed of propagation of a wavefro ...
. Cherenkov radiation is produced by charged particles which are traveling through a dielectric
In electromagnetism, a dielectric (or dielectric medium) is an Insulator (electricity), electrical insulator that can be Polarisability, polarised by an applied electric field. When a dielectric material is placed in an electric field, electric ...
substance at a speed greater than the speed of light
The speed of light in vacuum, commonly denoted , is a universal physical constant exactly equal to ). It is exact because, by international agreement, a metre is defined as the length of the path travelled by light in vacuum during a time i ...
in that medium. Despite the production of similarity-colored light and an association with high-energy particles, Cherenkov radiation is generated by a fundamentally different mechanism.
See also
*Airglow
Airglow is a faint emission of light by a planetary atmosphere. In the case of Earth's atmosphere, this optical phenomenon causes the night sky never to be completely dark, even after the effects of starlight and diffuse sky radiation, diffuse ...
References
{{reflist, 30em Atmospheric optical phenomena Electrical breakdown Ionosphere Plasma phenomena Radioactivity