Iodine Isotope on:
[Wikipedia]
[Google]
[Amazon]
There are 40 known 
550 ns, β−? , 147Xe , rowspan=3, 3/2−# , rowspan=3, , - , β−, n? , 146Xe , - , β−, 2n? , 145Xe
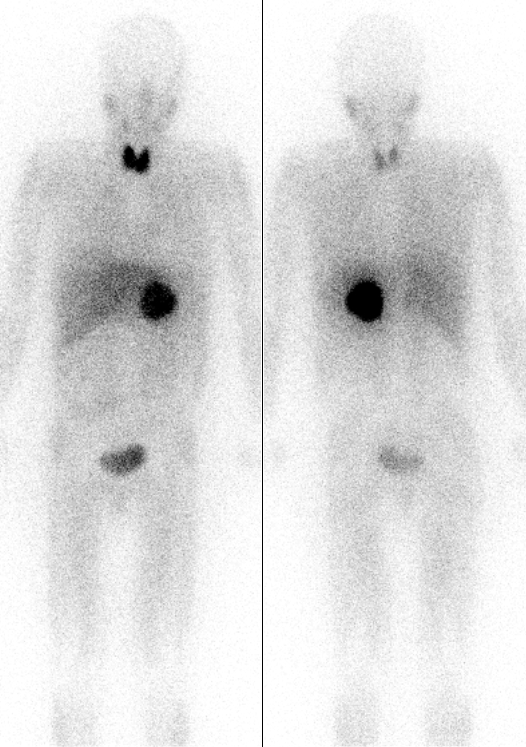 Iodine-131 () is a beta-emitting isotope with a half-life of eight days, and comparatively energetic (190 keV average and 606 keV maximum energy) beta radiation, which penetrates 0.6 to 2.0 mm from the site of uptake. This beta radiation can be used for the destruction of
Iodine-131 () is a beta-emitting isotope with a half-life of eight days, and comparatively energetic (190 keV average and 606 keV maximum energy) beta radiation, which penetrates 0.6 to 2.0 mm from the site of uptake. This beta radiation can be used for the destruction of
Iodine isotopes data from ''The Berkeley Laboratory Isotopes Project's''Iodine-128, Iodine-130, Iodine-132 data from 'Wolframalpha'
{{Authority control Iodine
isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s of iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
(53I) from 108I to 147I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element
A monoisotopic element is an element which has only a single stable isotope (nuclide). There are 26 such elements, as listed.
Stability is experimentally defined for chemical elements, as there are a number of stable nuclides with atomic number ...
.
Its longest-lived radioactive isotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
, 129I, has a half-life of 16.14 million years, which is too short for it to exist as a primordial nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
. Cosmogenic
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides (isotopes) created when a high-energy cosmic ray interacts with the nucleus of an ''in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the atom ( ...
sources of 129I produce very tiny quantities of it that are too small to affect atomic weight measurements; iodine is thus also a mononuclidic element
A mononuclidic element or monotopic element is one of the 21 chemical elements that is found naturally on Earth essentially as a single nuclide (which may, or may not, be a stable nuclide). This single nuclide will have a characteristic atomic ...
—one that is found in nature only as a single nuclide. Most 129I derived radioactivity on Earth is man-made, an unwanted long-lived byproduct of early nuclear tests and nuclear fission accidents.
All other iodine radioisotopes have half-lives less than 60 days, and four of these are used as tracers and therapeutic agents in medicine - 123I, 124I, 125I, and 131I. All industrial use of radioactive iodine isotopes involves these four.
The isotope 135I has a half-life less than seven hours, which is inconveniently short for those purposes. However, the unavoidable ''in situ'' production of this isotope is important in nuclear reactor control, as it decays to 135Xe, the most powerful known neutron absorber
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable ef ...
, and the nuclide
Nuclides (or nucleides, from nucleus, also known as nuclear species) are a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by the A ...
responsible for the so-called iodine pit
The iodine pit, also called the iodine hole or xenon pit, is a temporary disabling of a nuclear reactor due to the buildup of short- lived nuclear poisons in the reactor core. The main isotope responsible is 135Xe, mainly produced by natural d ...
phenomenon.
In addition to commercial production, 131I (half-life 8 days) is one of the common radioactive fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
s of nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
, and thus occurs in large amounts inside nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s. Due to its volatility, short half-life, and high abundance in fission products, 131I (along with the short-lived iodine isotope 132I, which is produced from the decay of 132Te with a half-life of 3 days) is responsible for the most dangerous part of the short-term radioactive contamination
Radioactive contamination, also called radiological pollution, is the deposition of, or presence of Radioactive decay, radioactive substances on surfaces or within solids, liquids, or gases (including the human body), where their presence is uni ...
after environmental release of the radioactive waste
Radioactive waste is a type of hazardous waste that contains radioactive material. It is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear ...
from a nuclear power plant. For that reason, iodine supplements (usually potassium iodide
Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are u ...
) are given to the populace after nuclear accidents or explosions (and in some cases prior to any such incident as a civil defense
Civil defense or civil protection is an effort to protect the citizens of a state (generally non-combatants) from human-made and natural disasters. It uses the principles of emergency management: Risk management, prevention, mitigation, prepara ...
mechanism) to reduce the uptake of radioactive iodine compounds by the thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans, it is a butterfly-shaped gland located in the neck below the Adam's apple. It consists of two connected lobes. The lower two thirds of the lobes are connected by ...
.
List of isotopes
, -id=Iodine-108 , rowspan=4, 108I , rowspan=4 style="text-align:right" , 53 , rowspan=4 style="text-align:right" , 55 , rowspan=4, 107.94335(11)# , rowspan=4, 26.4(8) ms , α (99.50%) , 104Sb , rowspan=4, 1+# , rowspan=4, , - , p (0.50%) , 107Te , - , β+? , 108Te , - , β+, p? , 107Sb , -id=Iodine-109 , rowspan=2, 109I , rowspan=2 style="text-align:right" , 53 , rowspan=2 style="text-align:right" , 56 , rowspan=2, 108.9380860(72) , rowspan=2, 92.8(8) μs , p (99.986%) , 108Te , rowspan=2, (1/2+,3/2+) , rowspan=2, , - , α (0.014%) , 105Sb , -id=Iodine-110 , rowspan=4, 110I , rowspan=4 style="text-align:right" , 53 , rowspan=4 style="text-align:right" , 57 , rowspan=4, 109.935085(66) , rowspan=4, 664(24) ms , β+ (71%) , 110Te , rowspan=4, (1+) , rowspan=4, , - , α (17%) , 106Sb , - , β+, p (11%) , 109Sb , - , β+, α (1.1%) , 106Sn , -id=Iodine-111 , rowspan=3, 111I , rowspan=3 style="text-align:right" , 53 , rowspan=3 style="text-align:right" , 58 , rowspan=3, 110.9302692(51) , rowspan=3, 2.5(2) s , β+ (99.91%) , 111Te , rowspan=3, 5/2+# , rowspan=3, , - , α (0.088%) , 107Sb , - , β+, p? , 110Sb , -id=Iodine-112 , rowspan=4, 112I , rowspan=4 style="text-align:right" , 53 , rowspan=4 style="text-align:right" , 59 , rowspan=4, 111.928005(11) , rowspan=4, 3.34(8) s , β+ (99.01%) , 112Te , rowspan=4, 1+# , rowspan=4, , - , β+, p (0.88%) , 111Sb , - , β+, α (0.104%) , 108Sn , - , α (0.0012%) , 108Sb , -id=Iodine-113 , rowspan=3, 113I , rowspan=3 style="text-align:right" , 53 , rowspan=3 style="text-align:right" , 60 , rowspan=3, 112.9236501(86) , rowspan=3, 6.6(2) s , β+ , 113Te , rowspan=3, 5/2+# , rowspan=3, , - , α (3.310×10−5#%) , 109Sb , - , β+, α? , 109Sn , -id=Iodine-114 , rowspan=3, 114I , rowspan=3 style="text-align:right" , 53 , rowspan=3 style="text-align:right" , 61 , rowspan=3, 113.922019(22) , rowspan=3, 2.01(15) s , β+ , 114Te , rowspan=3, 1+ , rowspan=3, , - , β+, p? , 113Sb , - , α (7.7×10−9#%) , 110Sb , -id=Iodine-114m , rowspan=2 style="text-indent:1em" , 114mI , rowspan=2 colspan="3" style="text-indent:2em" , 265.9(5) keV , rowspan=2, 6.2(5) s , β+? , 114Te , rowspan=2, (7−) , rowspan=2, , - , IT? , 114I , -id=Iodine-115 , 115I , style="text-align:right" , 53 , style="text-align:right" , 62 , 114.918048(31) , 1.3(2) min , β+ , 115Te , 5/2+# , , -id=Iodine-116 , 116I , style="text-align:right" , 53 , style="text-align:right" , 63 , 115.916886(81) , 2.91(15) s , β+ , 116Te , 1+ , , -id=Iodine-116m , style="text-indent:1em" , 116mI , colspan="3" style="text-indent:2em" , 430.4(5) keV , 3.27(16) μs , IT , 116I , (7−) , , -id=Iodine-117 , rowspan=2, 117I , rowspan=2 style="text-align:right" , 53 , rowspan=2 style="text-align:right" , 64 , rowspan=2, 116.913646(27) , rowspan=2, 2.22(4) min , β+ (77%) , 117Te , rowspan=2, (5/2)+ , rowspan=2, , - , EC (23%) , 117Te , -id=Iodine-118 , 118I , style="text-align:right" , 53 , style="text-align:right" , 65 , 117.913074(21) , 13.7(5) min , β+ , 118Te , (2−) , , -id=Iodine-118m , rowspan=2 style="text-indent:1em" , 118mI , rowspan=2 colspan="3" style="text-indent:2em" , 188.8(7) keV , rowspan=2, 8.5(5) min , β+ , 118Te , rowspan=2, (7−) , rowspan=2, , - , IT? , 118I , -id=Iodine-119 , rowspan=2, 119I , rowspan=2 style="text-align:right" , 53 , rowspan=2 style="text-align:right" , 66 , rowspan=2, 118.910061(23) , rowspan=2, 19.1(4) min , β+ (51%) , 119Te , rowspan=2, 5/2+ , rowspan=2, , - , EC (49%) , 119Te , -id=Iodine-120 , 120I , style="text-align:right" , 53 , style="text-align:right" , 67 , 119.910094(16) , 81.67(18) min , β+ , 120Te , 2− , , -id=Iodine-120m1 , style="text-indent:1em" , 120m1I , colspan="3" style="text-indent:2em" , 72.61(9) keV , 242(5) ns , IT , 120I , 3+ , , -id=Iodine-120m2 , style="text-indent:1em" , 120m2I , colspan="3" style="text-indent:2em" , 320(150) keV , 53(4) min , β+ , 120Te , (7−) , , -id=Iodine-121 , 121I , style="text-align:right" , 53 , style="text-align:right" , 68 , 120.9074115(51) , 2.12(1) h , β+ , 121Te , 5/2+ , , -id=Iodine-121m , style="text-indent:1em" , 121mI , colspan="3" style="text-indent:2em" , 2376.9(4) keV , 9.0(14) μs , IT , 121I , 21/2+# , , -id=Iodine-122 , rowspan=2, 122I , rowspan=2 style="text-align:right" , 53 , rowspan=2 style="text-align:right" , 69 , rowspan=2, 121.9075901(56) , rowspan=2, 3.63(6) min , β+ (78%) , 122Te , rowspan=2, 1+ , rowspan=2, , - , EC (22%) , 122Te , -id=Iodine-122m1 , style="text-indent:1em" , 122m1I , colspan="3" style="text-indent:2em" , 314.9(4) keV , 193.3(9) ns , IT , 122I , 7− , , -id=Iodine-122m2 , style="text-indent:1em" , 122m2I , colspan="3" style="text-indent:2em" , 379.4(5) keV , 79.1(12) μs , IT , 122I , 7− , , -id=Iodine-122m3 , style="text-indent:1em" , 122m3I , colspan="3" style="text-indent:2em" , 394.1(5) keV , 78.2(4) μs , IT , 122I , (8+) , , -id=Iodine-122m4 , style="text-indent:1em" , 122m4I , colspan="3" style="text-indent:2em" , 444.1(5) keV , 146.5(12) ns , IT , 122I , 8− , , - , 123IHas medical uses , style="text-align:right" , 53 , style="text-align:right" , 70 , 122.9055898(40) , 13.2232(15) h , EC , 123Te , 5/2+ , , - , 124I , style="text-align:right" , 53 , style="text-align:right" , 71 , 123.9062103(25) , 4.1760(3) d , β+ , 124Te , 2− , , - , 125I , style="text-align:right" , 53 , style="text-align:right" , 72 , 124.9046306(15) , 59.392(8) d , EC , 125Te , 5/2+ , , -id=Iodine-126 , rowspan=2, 126I , rowspan=2 style="text-align:right" , 53 , rowspan=2 style="text-align:right" , 73 , rowspan=2, 125.9056242(41) , rowspan=2, 12.93(5) d , β+ (52.7%) , 126Te , rowspan=2, 2− , rowspan=2, , - , β− (47.3%) , 126Xe , -id=Iodine-126m , style="text-indent:1em" , 126mI , colspan="3" style="text-indent:2em" , 111.00(23) keV , 128 ns , IT , 126I , 3+ , , -id=Iodine-127 , 127IFission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
, style="text-align:right" , 53
, style="text-align:right" , 74
, 126.9044726(39)
, colspan=3 align=center, Stable
, 5/2+
, 1.0000
, -
, rowspan=2, 128I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 75
, rowspan=2, 127.9058094(39)
, rowspan=2, 24.99(2) min
, β− (93.1%)
, 128Xe
, rowspan=2, 1+
, rowspan=2,
, -
, β+ (6.9%)
, ''128Te''
, -id=Iodine-128m1
, style="text-indent:1em" , 128m1I
, colspan="3" style="text-indent:2em" , 137.851(3) keV
, 845(20) ns
, IT
, 128I
, 4−
,
, -id=Iodine-128m2
, style="text-indent:1em" , 128m2I
, colspan="3" style="text-indent:2em" , 167.368(4) keV
, 175(15) ns
, IT
, 128I
, (6)−
,
, -
, 129ILong-lived fission product
Long-lived fission products (LLFPs) are radioactive materials with a long half-life (more than 200,000 years) produced by nuclear fission of uranium and plutonium. Because of their persistent radiotoxicity, it is necessary to isolate them from hum ...
Can be used to date certain early events in Solar System history and some use for dating groundwater
, style="text-align:right" , 53
, style="text-align:right" , 76
, 128.9049836(34)
, 1.614(12)×107 y
, β−
, 129Xe
, 7/2+
, TraceCosmogenic nuclide
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides (isotopes) created when a high-energy cosmic ray interacts with the nucleus of an '' in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the atom ...
, also found as nuclear contamination
, -id=Iodine-130
, 130I
, style="text-align:right" , 53
, style="text-align:right" , 77
, 129.9066702(34)
, 12.36(1) h
, β−
, 130Xe
, 5+
,
, -id=Iodine-130m1
, rowspan=2 style="text-indent:1em" , 130m1I
, rowspan=2 colspan="3" style="text-indent:2em" , 39.9525(13) keV
, rowspan=2, 8.84(6) min
, IT (84%)
, 130I
, rowspan=2, 2+
, rowspan=2,
, -
, β− (16%)
, 130Xe
, -id=Iodine-130m2
, style="text-indent:1em" , 130m2I
, colspan="3" style="text-indent:2em" , 69.5865(7) keV
, 133(7) ns
, IT
, 130I
, 6−
,
, -id=Iodine-130m3
, style="text-indent:1em" , 130m3I
, colspan="3" style="text-indent:2em" , 82.3960(19) keV
, 315(15) ns
, IT
, 130I
, (8−)
,
, -id=Iodine-130m4
, style="text-indent:1em" , 130m4I
, colspan="3" style="text-indent:2em" , 85.1099(10) keV
, 254(4) ns
, IT
, 130I
, 6−
,
, -
, 131I
, style="text-align:right" , 53
, style="text-align:right" , 78
, 130.90612638(65)
, 8.0249(6) d
, β−
, 131Xe
, 7/2+
,
, -id=Iodine-131m
, style="text-indent:1em" , 131mI
, colspan="3" style="text-indent:2em" , 1918.4(4) keV
, 24(1) μs
, IT
, 131I
, 19/2−
,
, -id=Iodine-132
, 132I
, style="text-align:right" , 53
, style="text-align:right" , 79
, 131.9079935(44)
, 2.295(13) h
, β−
, 132Xe
, 4+
,
, -id=Iodine-132m
, rowspan=2 style="text-indent:1em" , 132mI
, rowspan=2 colspan="3" style="text-indent:2em" , 110(11) keV
, rowspan=2, 1.387(15) h
, IT (86%)
, 132I
, rowspan=2, (8−)
, rowspan=2,
, -
, β− (14%)
, 132Xe
, -id=Iodine-133
, 133I
, style="text-align:right" , 53
, style="text-align:right" , 80
, 132.9078284(63)
, 20.83(8) h
, β−
, 133Xe
, 7/2+
,
, -id=Iodine-133m1
, style="text-indent:1em" , 133m1I
, colspan="3" style="text-indent:2em" , 1634.148(10) keV
, 9(2) s
, IT
, 133I
, (19/2−)
,
, -id=Iodine-133m2
, style="text-indent:1em" , 133m2I
, colspan="3" style="text-indent:2em" , 1729.137(10) keV
, ~170 ns
, IT
, 133I
, (15/2−)
,
, -id=Iodine-133m3
, style="text-indent:1em" , 133m3I
, colspan="3" style="text-indent:2em" , 2493.7(4) keV
, 469(15) ns
, IT
, 133I
, (23/2+)
,
, -id=Iodine-134
, 134I
, style="text-align:right" , 53
, style="text-align:right" , 81
, 133.9097757(52)
, 52.5(2) min
, β−
, 134Xe
, (4)+
,
, -id=Iodine-134m
, rowspan=2 style="text-indent:1em" , 134mI
, rowspan=2 colspan="3" style="text-indent:2em" , 316.49(22) keV
, rowspan=2, 3.52(4) min
, IT (97.7%)
, 134I
, rowspan=2, (8)−
, rowspan=2,
, -
, β− (2.3%)
, 134Xe
, -
, 135IFission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
that, along with its decay product 135Xe, is responsible for the iodine pit
The iodine pit, also called the iodine hole or xenon pit, is a temporary disabling of a nuclear reactor due to the buildup of short- lived nuclear poisons in the reactor core. The main isotope responsible is 135Xe, mainly produced by natural d ...
instability in nuclear reactors
, style="text-align:right" , 53
, style="text-align:right" , 82
, 134.9100594(22)
, 6.58(3) h
, β−
, 135Xe
, 7/2+
,
, -id=Iodine-136
, 136I
, style="text-align:right" , 53
, style="text-align:right" , 83
, 135.914605(15)
, 83.4(4) s
, β−
, ''136Xe''
, (1−)
,
, -id=Iodine-136m
, style="text-indent:1em" , 136mI
, colspan="3" style="text-indent:2em" , 206(15) keV
, 46.6(11) s
, β−
, ''136Xe''
, (6−)
,
, -id=Iodine-137
, rowspan=2, 137I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 84
, rowspan=2, 136.9180282(90)
, rowspan=2, 24.13(12) s
, β− (92.49%)
, 137Xe
, rowspan=2, 7/2+#
, rowspan=2,
, -
, β−, n (7.51%)
, ''136Xe''
, -id=Iodine-138
, rowspan=2, 138I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 85
, rowspan=2, 137.9227264(64)
, rowspan=2, 6.26(3) s
, β− (94.67%)
, 138Xe
, rowspan=2, (1−)
, rowspan=2,
, -
, β−, n (5.33%)
, 137Xe
, -id=Iodine-138m
, style="text-indent:1em" , 138mI
, colspan="3" style="text-indent:2em" , 67.9(3) keV
, 1.26(16) μs
, IT
, 138I
, (3−)
,
, -id=Iodine-139
, rowspan=2, 139I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 86
, rowspan=2, 138.9264934(43)
, rowspan=2, 2.280(11) s
, β− (90.26%)
, 139Xe
, rowspan=2, 7/2+#
, rowspan=2,
, -
, β−, n (9.74%)
, 138Xe
, -id=Iodine-140
, rowspan=3, 140I
, rowspan=3 style="text-align:right" , 53
, rowspan=3 style="text-align:right" , 87
, rowspan=3, 139.931716(13)
, rowspan=3, 588(10) ms
, β− (92.40%)
, 140Xe
, rowspan=3, (2−)
, rowspan=3,
, -
, β−, n (7.60%)
, 139Xe
, -
, β−, 2n?
, 138Xe
, -id=Iodine-141
, rowspan=2, 141I
, rowspan=2 style="text-align:right" , 53
, rowspan=2 style="text-align:right" , 88
, rowspan=2, 140.935666(17)
, rowspan=2, 420(7) ms
, β− (78.8%)
, 141Xe
, rowspan=2, 7/2+#
, rowspan=2,
, -
, β−, n (21.2%)
, 140Xe
, -id=Iodine-142
, rowspan=3, 142I
, rowspan=3 style="text-align:right" , 53
, rowspan=3 style="text-align:right" , 89
, rowspan=3, 141.9411666(53)
, rowspan=3, 235(11) ms
, β−
, 142Xe
, rowspan=3, 2−#
, rowspan=3,
, -
, β−, n?
, 141Xe
, -
, β−, 2n?
, 140Xe
, -id=Iodine-143
, rowspan=3, 143I
, rowspan=3 style="text-align:right" , 53
, rowspan=3 style="text-align:right" , 90
, rowspan=3, 142.94548(22)#
, rowspan=3, 182(8) ms
, β−
, 143Xe
, rowspan=3, 7/2+#
, rowspan=3,
, -
, β−, n?
, 142Xe
, -
, β−, 2n?
, 141Xe
, -id=Iodine-144
, rowspan=3, 144I
, rowspan=3 style="text-align:right" , 53
, rowspan=3 style="text-align:right" , 91
, rowspan=3, 143.95134(43)#
, rowspan=3, 94(8) ms
, β−
, 144Xe
, rowspan=3, 1−#
, rowspan=3,
, -
, β−, n?
, 143Xe
, -
, β−, 2n?
, 142Xe
, -id=Iodine-145
, rowspan=3, 145I
, rowspan=3 style="text-align:right" , 53
, rowspan=3 style="text-align:right" , 92
, rowspan=3, 144.95585(54)#
, rowspan=3, 89.7(93) ms
, β−
, 145Xe
, rowspan=3, 7/2+#
, rowspan=3,
, -
, β−, n?
, 144Xe
, -
, β−, 2n?
, 143Xe
, -id=Iodine-146
, rowspan=3, 146I
, rowspan=3 style="text-align:right" , 53
, rowspan=3 style="text-align:right" , 93
, rowspan=3, 145.96185(32)#
, rowspan=3, 94(26) ms
, β−
, 146Xe
, rowspan=3,
, rowspan=3,
, -
, β−, n?
, 145Xe
, -
, β−, 2n?
, 144Xe
, -id=Iodine-147
, rowspan=3, 147I
, rowspan=3 style="text-align:right" , 53
, rowspan=3 style="text-align:right" , 94
, rowspan=3, 146.96651(32)#
, rowspan=3, 60# ms550 ns, β−? , 147Xe , rowspan=3, 3/2−# , rowspan=3, , - , β−, n? , 146Xe , - , β−, 2n? , 145Xe
Notable radioisotopes
Radioisotopes of iodine are called radioactive iodine or radioiodine. Dozens exist, but about a half dozen are the most notable inapplied science
Applied science is the application of the scientific method and scientific knowledge to attain practical goals. It includes a broad range of disciplines, such as engineering and medicine. Applied science is often contrasted with basic science, ...
s such as the life sciences and nuclear power, as detailed below. Mentions of radioiodine in health care
Health care, or healthcare, is the improvement or maintenance of health via the preventive healthcare, prevention, diagnosis, therapy, treatment, wikt:amelioration, amelioration or cure of disease, illness, injury, and other disability, physic ...
contexts refer more often to iodine-131 than to other isotopes.
Of the many isotopes of iodine, only two are typically used in a medical setting: iodine-123 and iodine-131. Since 131I has both a beta and gamma decay mode, it can be used for radiotherapy or for imaging. 123I, which has no beta activity, is more suited for routine nuclear medicine imaging of the thyroid and other medical processes and less damaging internally to the patient. There are some situations in which iodine-124 and iodine-125 are also used in medicine.
Due to preferential uptake of iodine by the thyroid, radioiodine is extensively used in imaging of and, in the case of 131I, destroying dysfunctional thyroid tissues. Other types of tissue selectively take up certain iodine-131-containing tissue-targeting and killing radiopharmaceutical agents (such as MIBG
Iobenguane, or MIBG, is an aralkylguanidine analog of the adrenergic neurotransmitter norepinephrine (noradrenaline), typically used as a radiopharmaceutical. It acts as a blocking agent for adrenergic neurons. When radiolabeled, it can be use ...
). Iodine-125 is the only other iodine radioisotope used in radiation therapy, but only as an implanted capsule in brachytherapy
Brachytherapy is a form of radiation therapy where a sealed radiation, radiation source is placed inside or next to the area requiring treatment. The word "brachytherapy" comes from the Ancient Greek, Greek word , meaning "short-distance" or "s ...
, where the isotope never has a chance to be released for chemical interaction with the body's tissues.
Iodine-123 and iodine-125
The gamma-emitting isotopes iodine-123 (half-life 13 hours), and (less commonly) the longer-lived and less energetic iodine-125 (half-life 59 days) are used asnuclear imaging
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', because it reco ...
tracers to evaluate the anatomic and physiologic function of the thyroid. Abnormal results may be caused by disorders such as Graves' disease
Graves' disease, also known as toxic diffuse goiter or Basedow's disease, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyro ...
or Hashimoto's thyroiditis
Hashimoto's thyroiditis, also known as chronic lymphocytic thyroiditis, Hashimoto's disease and autoimmune thyroiditis, is an autoimmune disease in which the thyroid gland is gradually destroyed.
Early on, symptoms may not be noticed. Over ti ...
. Both isotopes decay by electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Th ...
(EC) to the corresponding tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fou ...
nuclides, but in neither case are these the metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
nuclides 123mTe and 125mTe (which are of higher energy, and are not produced from radioiodine). Instead, the excited tellurium nuclides decay immediately (half-life too short to detect). Following EC, the excited 123Te from 123I emits a high-speed 127 keV internal conversion
Internal conversion is an atomic decay process where an excited nucleus interacts electromagnetically with one of the orbital electrons of an atom. This causes the electron to be emitted (ejected) from the atom. Thus, in internal conversion (o ...
electron (not a beta ray
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus, known as beta decay. There are two forms of beta decay, β− decay and � ...
) about 13% of the time, but this does little cellular damage due to the nuclide's short half-life and the relatively small fraction of such events. In the remainder of cases, a 159 keV gamma ray is emitted, which is well-suited for gamma imaging.
Excited 125Te resulting from electron capture of 125I also emits a much lower-energy internal conversion electron (35.5 keV), which does relatively little damage due to its low energy, even though its emission is more common. The relatively low-energy gamma from 125I/125Te decay is poorly suited for imaging, but can still be seen, and this longer-lived isotope is necessary in tests that require several days of imaging, for example, fibrinogen scan imaging to detect blood clots.
Both 123I and 125I emit copious low energy Auger electron
The Auger effect (; ) or Meitner-Auger effect is a physical phenomenon in which atoms eject electrons. It occurs when an inner-shell vacancy in an atom is filled by an electron, releasing energy that causes the emission of another electron from a ...
s after their decay, but these do not cause serious damage (double-stranded DNA breaks) in cells, unless the nuclide is incorporated into a medication that accumulates in the nucleus, or into DNA (this is never the case is clinical medicine, but it has been seen in experimental animal models).
Iodine-125 is also commonly used by radiation oncologists in low dose rate brachytherapy
Brachytherapy is a form of radiation therapy where a sealed radiation, radiation source is placed inside or next to the area requiring treatment. The word "brachytherapy" comes from the Ancient Greek, Greek word , meaning "short-distance" or "s ...
in the treatment of cancer at sites other than the thyroid, especially in prostate cancer
Prostate cancer is the neoplasm, uncontrolled growth of cells in the prostate, a gland in the male reproductive system below the bladder. Abnormal growth of the prostate tissue is usually detected through Screening (medicine), screening tests, ...
. When 125I is used therapeutically, it is encapsulated in titanium seeds and implanted in the area of the tumor, where it remains. The low energy of the gamma spectrum in this case limits radiation damage to tissues far from the implanted capsule. Iodine-125, due to its suitable longer half-life and less penetrating gamma spectrum, is also often preferred for laboratory tests that rely on iodine as a tracer that is counted by a gamma counter
A gamma counter is an instrument to measure gamma radiation emitted by a radionuclide. Unlike survey meters, gamma counters are designed to measure small samples of radioactive material, typically with automated measurement and movement of multip ...
, such as in radioimmunoassay
A radioimmunoassay (RIA) is an immunoassay that uses radioactive tracer, radiolabeled molecules in a stepwise formation of immune complexes. A RIA is a very sensitive in vitro assay technique used to measure concentrations of substances, usually m ...
ing.
I is used as the radiolabel
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide (a radioactive atom). By virtue of its radioactive decay, it can be used to exp ...
in investigating which ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s go to which plant pattern recognition receptor
Plants are the eukaryotes that form the kingdom Plantae; they are predominantly photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with cyanobacteria to produce sugars fro ...
s (PRRs).
Iodine-124
Iodine-124 is a proton-rich isotope of iodine with a half-life of 4.18 days. Its modes of decay are: 74.4% electron capture, 25.6% positron emission. 124I decays to 124Te. Iodine-124 can be made by numerous nuclear reactions via acyclotron
A cyclotron is a type of particle accelerator invented by Ernest Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Januar ...
. The most common starting material used is 124Te.
Iodine-124 as the iodide salt can be used to directly image the thyroid using positron emission tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, r ...
(PET). Iodine-124 can also be used as a PET radiotracer
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide (a radioactive atom). By virtue of its radioactive decay, it can be used to ...
with a usefully longer half-life compared with fluorine-18
Fluorine-18 (18F, also called radiofluorine) is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380(6) u and its half-life is 109.771(20) minutes. It decays by positron emission 96.7% of the time and el ...
. In this use, the nuclide is chemically bonded to a pharmaceutical to form a positron-emitting radiopharmaceutical, and injected into the body, where again it is imaged by PET scan.
Iodine-129
Iodine-129 (129I;half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
15.7 million years) is a product of cosmic ray spallation
Cosmic ray spallation, also known as the x-process, is a set of naturally occurring nuclear reactions causing nucleosynthesis; it refers to the formation of chemical elements from the impact of cosmic rays on an object. Cosmic rays are highly ene ...
on various isotopes of xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
in the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
, in cosmic ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the ...
muon
A muon ( ; from the Greek letter mu (μ) used to represent it) is an elementary particle similar to the electron, with an electric charge of −1 '' e'' and a spin of ''ħ'', but with a much greater mass. It is classified as a ...
interaction with tellurium-130, and also uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
and plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
fission, both in subsurface rocks and nuclear reactors. Artificial nuclear processes, in particular nuclear fuel reprocessing and atmospheric nuclear weapons tests, have now swamped the natural signal for this isotope. Nevertheless, it now serves as a groundwater tracer as indicator of nuclear waste dispersion into the natural environment. In a similar fashion, 129I was used in rainwater studies to track fission products following the Chernobyl disaster
On 26 April 1986, the no. 4 reactor of the Chernobyl Nuclear Power Plant, located near Pripyat, Ukrainian Soviet Socialist Republic, Ukrainian SSR, Soviet Union (now Ukraine), exploded. With dozens of direct casualties, it is one of only ...
.
In some ways, 129I is similar to 36Cl. It is a soluble halogen, exists mainly as a non-sorbing anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
, and is produced by cosmogenic, thermonuclear, and in-situ reactions. In hydrologic studies, 129I concentrations are usually reported as the ratio of 129I to total I (which is virtually all 127I). As is the case with 36Cl/Cl, 129I/I ratios in nature are quite small, 10−14 to 10−10 (peak thermonuclear 129I/I during the 1960s and 1970s reached about 10−7). 129I differs from 36Cl in that its half-life is longer (15.7 vs. 0.301 million years), it is highly biophilic, and occurs in multiple ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ic forms (commonly, I− and IO3−), which have different chemical behaviors. This makes it fairly easy for 129I to enter the biosphere as it becomes incorporated into vegetation, soil, milk, animal tissue, etc.
Excesses of stable 129Xe in meteorites have been shown to result from decay of "primordial" iodine-129 produced newly by the supernovas that created the dust and gas from which the Solar System formed. This isotope has long decayed and is thus referred to as "extinct". Historically, 129I was the first extinct radionuclide
An extinct radionuclide is a radionuclide that was formed by nucleosynthesis before the formation of the Solar System, about 4.6 billion years ago, but has since decayed to virtually zero abundance and is no longer detectable as a primordial nu ...
to be identified as present in the early Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Sola ...
. Its decay is the basis of the I-Xe iodine-xenon radiometric dating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to Chronological dating, date materials such as Rock (geology), rocks or carbon, in which trace radioactive impurity, impurities were selectively incorporat ...
scheme, which covers the first 85 million years of Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Sola ...
evolution.
Iodine-131
 Iodine-131 () is a beta-emitting isotope with a half-life of eight days, and comparatively energetic (190 keV average and 606 keV maximum energy) beta radiation, which penetrates 0.6 to 2.0 mm from the site of uptake. This beta radiation can be used for the destruction of
Iodine-131 () is a beta-emitting isotope with a half-life of eight days, and comparatively energetic (190 keV average and 606 keV maximum energy) beta radiation, which penetrates 0.6 to 2.0 mm from the site of uptake. This beta radiation can be used for the destruction of thyroid nodule
Thyroid nodules are nodules (raised areas of tissue or fluid) which commonly arise within an otherwise normal thyroid gland. They may be hyperplastic or tumorous, but only a small percentage of thyroid tumors are malignant. Small, asymptomatic ...
s or hyperfunctioning thyroid tissue and for elimination of remaining thyroid tissue after surgery for the treatment of Graves' disease
Graves' disease, also known as toxic diffuse goiter or Basedow's disease, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyro ...
. The purpose of this therapy, which was first explored by Dr. Saul Hertz in 1941, is to destroy thyroid tissue that could not be removed surgically. In this procedure, 131I is administered either intravenously or orally following a diagnostic scan. This procedure may also be used, with higher doses of radio-iodine, to treat patients with thyroid cancer
Thyroid cancer is cancer that develops from the tissues of the thyroid gland. It is a disease in which cells grow abnormally and have the potential to spread to other parts of the body. Symptoms can include swelling or a lump in the neck, ...
.
The 131I is taken up into thyroid tissue and concentrated there. The beta particles emitted by the radioisotope destroys the associated thyroid tissue with little damage to surrounding tissues (more than 2.0 mm from the tissues absorbing the iodine). Due to similar destruction, 131I is the iodine radioisotope used in other water-soluble iodine-labeled radiopharmaceutical
Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which ...
s (such as MIBG
Iobenguane, or MIBG, is an aralkylguanidine analog of the adrenergic neurotransmitter norepinephrine (noradrenaline), typically used as a radiopharmaceutical. It acts as a blocking agent for adrenergic neurons. When radiolabeled, it can be use ...
) used therapeutically to destroy tissues.
The high energy beta radiation (up to 606 keV) from 131I causes it to be the most carcinogenic of the iodine isotopes. It is thought to cause the majority of excess thyroid cancers seen after nuclear fission contamination (such as bomb fallout or severe nuclear reactor accidents like the Chernobyl disaster
On 26 April 1986, the no. 4 reactor of the Chernobyl Nuclear Power Plant, located near Pripyat, Ukrainian Soviet Socialist Republic, Ukrainian SSR, Soviet Union (now Ukraine), exploded. With dozens of direct casualties, it is one of only ...
) However, these epidemiological effects are seen primarily in children, and treatment of adults and children with therapeutic 131I, and epidemiology of adults exposed to low-dose 131I has not demonstrated carcinogenicity.
Iodine-135
Iodine-135 is an isotope of iodine with a half-life of 6.6 hours. It is an important isotope from the viewpoint ofnuclear reactor physics Nuclear reactor physics is the field of physics that studies and deals with the applied study and engineering applications of chain reaction to induce a controlled rate of fission in a nuclear reactor for the production of energy.van Dam, H., van d ...
. It is produced in relatively large amounts as a fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
, and decays to xenon-135
Xenon-135 (135Xe) is an Isotope#Radioactive, primordial, and stable isotopes, unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and it is the most powerful known neutron-absorbing nuclear poison ...
, which is a nuclear poison
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable ef ...
with the largest known thermal neutron cross section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of ...
, which is a cause of multiple complications in the control of nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s. The process of buildup of xenon-135
Xenon-135 (135Xe) is an Isotope#Radioactive, primordial, and stable isotopes, unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and it is the most powerful known neutron-absorbing nuclear poison ...
from accumulated iodine-135 can temporarily preclude a shut-down reactor from restarting. This is known as xenon poisoning or "falling into an iodine pit
The iodine pit, also called the iodine hole or xenon pit, is a temporary disabling of a nuclear reactor due to the buildup of short- lived nuclear poisons in the reactor core. The main isotope responsible is 135Xe, mainly produced by natural d ...
".
Nonradioactive iodide (127I) as protection from unwanted radioiodine uptake by the thyroid
Colloquially, radioactive materials can be described as "hot," and non-radioactive materials can be described as "cold." There are instances in which cold iodide is administered to people in order to prevent the uptake of hot iodide by the thyroid gland. For example, blockage of thyroid iodine uptake with potassium iodide is used innuclear medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', ...
scintigraphy
Scintigraphy (from Latin ''scintilla'', "spark"), also known as a gamma scan, is a diagnostic test in nuclear medicine, where radioisotopes attached to drugs that travel to a specific organ or tissue (radiopharmaceuticals) are taken internally a ...
and therapy with some radioiodinated compounds that are not targeted to the thyroid, such as iobenguane
Iobenguane, or MIBG, is an aralkylguanidine analog of the adrenergic neurotransmitter norepinephrine (noradrenaline), typically used as a radiopharmaceutical. It acts as a blocking agent for adrenergic neurons. When radiolabeled, it can be use ...
(MIBG
Iobenguane, or MIBG, is an aralkylguanidine analog of the adrenergic neurotransmitter norepinephrine (noradrenaline), typically used as a radiopharmaceutical. It acts as a blocking agent for adrenergic neurons. When radiolabeled, it can be use ...
), which is used to image or treat neural tissue tumors, or iodinated fibrinogen, which is used in fibrinogen scans to investigate clotting. These compounds contain iodine, but not in the iodide form. However, since they may be ultimately metabolized or break down to radioactive iodide, it is common to administer non-radioactive potassium iodide to insure that metabolites of these radiopharmaceuticals is not sequestered by the thyroid gland and result in a concentrated radiation dose to that tissue.
Potassium iodide
Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are u ...
has been distributed to populations exposed to nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
accidents such as the Chernobyl disaster
On 26 April 1986, the no. 4 reactor of the Chernobyl Nuclear Power Plant, located near Pripyat, Ukrainian Soviet Socialist Republic, Ukrainian SSR, Soviet Union (now Ukraine), exploded. With dozens of direct casualties, it is one of only ...
. The iodide solution SSKI, a saturated solution of potassium (K) iodide in water, has been used to block absorption of the radioiodine. Tablets containing potassium iodide are now also manufactured and stocked in central disaster sites by some governments for this purpose. In theory, many cancers might be prevented in this way, since an excess of thyroid cancer, presumably due to radioiodine uptake, is the only proven long-term effect after exposure of a population to radioactive fission products from a reactor accident or atomic bomb fallout. Taking large amounts of iodide saturates thyroid receptors and prevents uptake of most radioactive iodine-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nu ...
that may be present from fission product exposure (although it does not protect from other radioisotopes, nor from any external radiation). The protective effect of KI lasts approximately 24 hours, so must be dosed daily until a risk of significant exposure to this isotope no longer exists; as it decays relatively rapidly with a half-life of eight days (other iodine isotopes of concern are even shorter-lived), 99.95% of the radioiodine has vanished after three months.
See also
Daughter products other than iodine *Isotopes of xenon
Naturally occurring xenon (54Xe) consists of seven stable isotopes and two very long-lived isotopes. Double electron capture has been observed in 124Xe (half-life ) and double beta decay in 136Xe (half-life ), which are among the longest measured ...
* Isotopes of tellurium
There are 39 known isotopes and 17 nuclear isomers of tellurium (52Te), with atomic masses that range from 104 to 142. These are listed in the table below.
Naturally-occurring tellurium on Earth consists of eight isotopes. Two of these have been ...
* Isotopes of antimony
Antimony (Sb) occurs in two stable isotopes, Sb and Sb. There are 37 artificial radioactive isotopes, the longest-lived of which are Sb, with a half-life of 2.75856 years; Sb, with half-life 60.2 days; and Sb, with half-life 12.35 days. All other ...
* Isotopes of tin
Tin (50Sn) is the element with the greatest number of stable isotopes (ten; three of them are potentially radioactive but have not been observed to decay). This is probably related to the fact that 50 is a " magic number" of protons. In addition, ...
References
* Isotope masses from: ** * Half-life, spin, and isomer data selected from the following sources. ** ** **External links
Iodine isotopes data from ''The Berkeley Laboratory Isotopes Project's''
{{Authority control Iodine
Iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
Nuclear safety and security