InsR on:
[Wikipedia]
[Google]
[Amazon]
The insulin receptor (IR) is a
 The insulin receptor's endogenous ligands include
The insulin receptor's endogenous ligands include
transmembrane receptor
Cell surface receptors (membrane receptors, transmembrane receptors) are receptor (biochemistry), receptors that are embedded in the cell membrane, plasma membrane of cell (biology), cells. They act in cell signaling by receiving (binding to) ex ...
that is activated by insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (''INS)'' gene. It is the main Anabolism, anabolic hormone of the body. It regulates the metabol ...
, IGF-I, IGF-II and belongs to the large class of receptor tyrosine kinase
Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinas ...
. Metabolically, the insulin receptor plays a key role in the regulation of glucose homeostasis
Blood sugar regulation is the process by which the levels of blood sugar, the common name for glucose dissolved in blood plasma, are maintained by the body within a narrow range. This tight regulation is referred to as glucose homeostasis. Insul ...
; a functional process that under degenerate conditions may result in a range of clinical manifestations including diabetes
Diabetes mellitus, commonly known as diabetes, is a group of common endocrine diseases characterized by sustained high blood sugar levels. Diabetes is due to either the pancreas not producing enough of the hormone insulin, or the cells of th ...
and cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
. Insulin signalling controls access to blood glucose in body cells. When insulin falls, especially in those with high insulin sensitivity, body cells begin only to have access to lipids that do not require transport across the membrane. So, in this way, insulin is the key regulator of fat metabolism as well. Biochemically, the insulin receptor is encoded by a single gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
, from which alternate splicing
Alternative splicing, alternative RNA splicing, or differential splicing, is an alternative splicing process during gene expression that allows a single gene to produce different splice variants. For example, some exons of a gene may be included ...
during transcription results in either IR-A or IR-B isoforms
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have uniqu ...
. Downstream post-translational events of either isoform result in the formation of a proteolytically cleaved α and β subunit, which upon combination are ultimately capable of homo or hetero-dimerisation to produce the ≈320 kDa disulfide-linked transmembrane insulin receptor.
Structure
Initially, transcription of alternative splice variants derived from the ''INSR'' gene aretranslated
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
to form one of two monomeric isomers; IR-A in which exon
An exon is any part of a gene that will form a part of the final mature RNA produced by that gene after introns have been removed by RNA splicing. The term ''exon'' refers to both the DNA sequence within a gene and to the corresponding sequence ...
11 is excluded, and IR-B in which exon 11 is included. Inclusion of exon 11 results in the addition of 12 amino acids upstream of the intrinsic furin proteolytic cleavage site.
Upon receptor dimerisation, after proteolytic cleavage
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Protein degradation is a major regulatory mechanism of gene expression and contributes substantially to shaping mammalian proteomes. Uncatalysed, the hydrolysis o ...
into the α- and β-chains, the additional 12 amino acids remain present at the C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein
Proteins are large biomolecules and macromolecules that comp ...
of the α-chain (designated αCT) where they are predicted to influence receptor–ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
interaction.
Each isometric monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
is structurally organized into 8 distinct domains consists of; a leucine-rich repeat domain (L1, residues 1–157), a cysteine-rich region (CR, residues 158–310), an additional leucine rich repeat domain (L2, residues 311–470), three fibronectin type III domain
The Fibronectin type III domain is an evolutionarily conserved protein domain that is widely found in animal proteins. The fibronectin protein in which this domain was first identified contains 16 copies of this domain. The domain is about 100 am ...
s; FnIII-1 (residues 471–595), FnIII-2 (residues 596–808) and FnIII-3 (residues 809–906). Additionally, an insert domain (ID, residues 638–756) resides within FnIII-2, containing the α/β furin cleavage site, from which proteolysis results in both IDα and IDβ domains. Within the β-chain, downstream of the FnIII-3 domain lies a transmembrane helix (TH) and intracellular juxtamembrane (JM) region, just upstream of the intracellular tyrosine kinase (TK) catalytic domain, responsible for subsequent intracellular signaling pathways.
Upon cleavage of the monomer to its respective α- and β-chains, receptor hetero or homo-dimerisation is maintained covalently between chains by a single disulphide link and between monomers in the dimer by two disulphide links extending from each α-chain. The overall 3D ectodomain
An ectodomain is the domain of a membrane protein that extends into the extracellular space (the space outside a cell). Ectodomains are usually the parts of proteins that initiate contact with surfaces, which leads to signal transduction. A n ...
structure, possessing four ligand binding sites, resembles an inverted 'V', with the each monomer rotated approximately 2-fold about an axis running parallel to the inverted 'V' and L2 and FnIII-1 domains from each monomer forming the inverted 'V's apex.
Ligand binding
 The insulin receptor's endogenous ligands include
The insulin receptor's endogenous ligands include insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (''INS)'' gene. It is the main Anabolism, anabolic hormone of the body. It regulates the metabol ...
, IGF-I and IGF-II. Using a cryo-EM
Cryogenic electron microscopy (cryo-EM) is a transmission electron microscopy technique applied to samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An ...
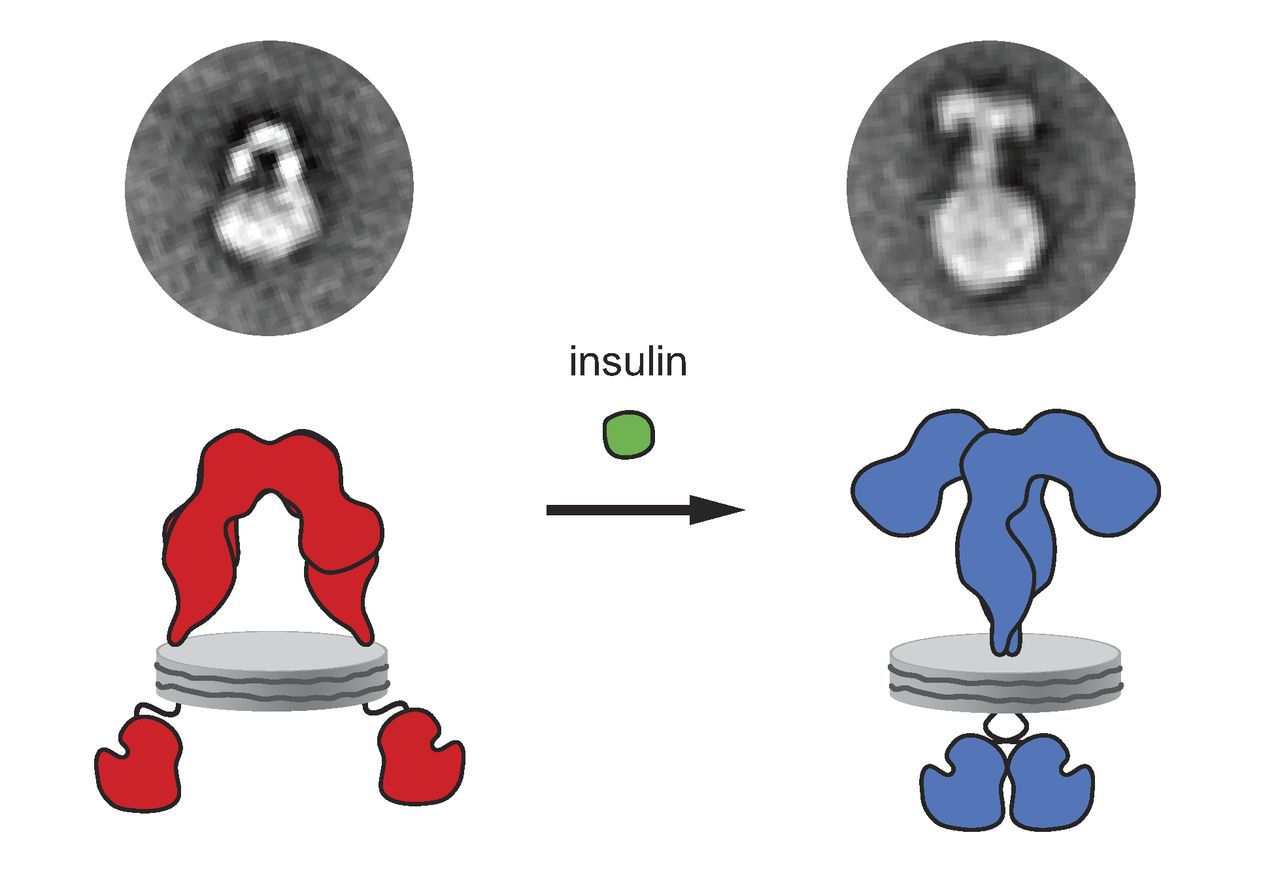
, structural insight into conformational changes upon insulin binding was provided. Binding of ligand to the α-chains of the IR dimeric ectodomain shifts it from an inverted V-shape to a T-shaped conformation, and this change is propagated structurally to the transmembrane domains, which get closer, eventually leading to autophosphorylation of various tyrosine residues within the intracellular TK domain of the β-chain. These changes facilitate the recruitment of specific adapter proteins such as the insulin receptor substrate proteins (IRS) in addition to SH2-B ( Src Homology 2 - B ), APS and protein phosphatases, such as PTP1B, eventually promoting downstream processes involving blood glucose homeostasis.
Strictly speaking the relationship between IR and ligand shows complex allosteric properties. This was indicated with the use of a Scatchard plots which identified that the measurement of the ratio of IR bound ligand to unbound ligand does not follow a linear relationship with respect to changes in the concentration of IR bound ligand, suggesting that the IR and its respective ligand share a relationship of cooperative binding
Cooperative binding occurs in molecular binding systems containing more than one type, or species, of molecule and in which one of the partners is not mono-valent and can bind more than one molecule of the other species. In general, molecular bindi ...
. Furthermore, the observation that the rate of IR-ligand dissociation is accelerated upon addition of unbound ligand implies that the nature of this cooperation is negative; said differently, that the initial binding of ligand to the IR inhibits further binding to its second active site - exhibition of allosteric inhibition.
These models state that each IR monomer possesses 2 insulin binding sites; site 1, which binds to the 'classical' binding surface of insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (''INS)'' gene. It is the main Anabolism, anabolic hormone of the body. It regulates the metabol ...
: consisting of L1 plus αCT domains and site 2, consisting of loops at the junction of FnIII-1 and FnIII-2 predicted to bind to the 'novel' hexamer face binding site of insulin. As each monomer contributing to the IR ectodomain exhibits 3D 'mirrored' complementarity, N-terminal site 1 of one monomer ultimately faces C-terminal site 2 of the second monomer, where this is also true for each monomers mirrored complement (the opposite side of the ectodomain structure). Current literature distinguishes the complement binding sites by designating the second monomer's site 1 and site 2 nomenclature as either site 3 and site 4 or as site 1' and site 2' respectively.
As such, these models state that each IR may bind to an insulin molecule (which has two binding surfaces) via 4 locations, being site 1, 2, (3/1') or (4/2'). As each site 1 proximally faces site 2, upon insulin binding to a specific site, 'crosslinking' via ligand between monomers is predicted to occur (i.e. as onomer 1 Site 1 - Insulin - monomer 2 Site (4/2')or as onomer 1 Site 2 - Insulin - monomer 2 site (3/1'). In accordance with current mathematical modelling of IR-insulin kinetics, there are two important consequences to the events of insulin crosslinking; 1. that by the aforementioned observation of negative cooperation between IR and its ligand that subsequent binding of ligand to the IR is reduced and 2. that the physical action of crosslinking brings the ectodomain into such a conformation that is required for intracellular tyrosine phosphorylation events to ensue (i.e. these events serve as the requirements for receptor activation and eventual maintenance of blood glucose homeostasis).
Visualization of full length IR complexes is not yet available due to many constrain. Visualization of full length IR–insulin complexes is not yet available due to flexible link of transmembrane (TM) domains with extracellular domain and intracellular domain. The transmembrane (TM) domains are critical for activation and downstream signaling. Stabilization of TM domains may be result of phosphatidylinositol. Meanwhile, visualization of full length IR–downstream proteins is challenging because of transient nature of association, the phosphorylation receptor requirement, and the unfixed relative orientation.
Applying cryo-EM and molecular dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the Motion (physics), physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamics ( ...
simulations of receptor reconstituted in nanodiscs, the structure of the entire dimeric insulin receptor ectodomain with four insulin molecules bound was visualized, therefore confirming and directly showing biochemically predicted 4 binding locations.
Agonists
* 4548-G05 *Insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (''INS)'' gene. It is the main Anabolism, anabolic hormone of the body. It regulates the metabol ...
* Insulin-like growth factor 1
Insulin-like growth factor 1 (IGF-1), also called somatomedin C, is a hormone similar in tertiary structure, molecular structure to insulin which plays an important role in childhood growth, and has Anabolism, anabolic effects in adults. In the ...
* Mecasermin
A number of small-molecule
In molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; t ...
insulin receptor agonists have been identified.
Signal transduction pathway
The insulin receptor is a type oftyrosine kinase receptor
Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase ...
, in which the binding of an agonistic ligand triggers autophosphorylation
Autophosphorylation is a type of post-translational modification of proteins. It is generally defined as the phosphorylation of the kinase by itself. In eukaryotes, this process occurs by the addition of a phosphate group to serine, threonine o ...
of the tyrosine residues, with each subunit phosphorylating its partner. The addition of the phosphate groups generates a binding site for the insulin receptor substrate (IRS-1), which is subsequently activated via phosphorylation. The activated IRS-1 initiates the signal transduction pathway and binds to phosphoinositide 3-kinase
Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which i ...
(PI3K), in turn causing its activation. This then catalyses the conversion of Phosphatidylinositol 4,5-bisphosphate
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)''P''2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)''P''2 is enriched at the plasma membrane where it is a substrate for a number of ...
into Phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 acts as a secondary messenger and induces the activation of phosphatidylinositol dependent protein kinase, which then activates several other kinases – most notably protein kinase B
Protein kinase B (PKB), also known as Akt, is the collective name of a set of three serine/threonine-specific protein kinases that play key roles in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, trans ...
, (PKB, also known as Akt). PKB triggers the translocation of glucose transporter (GLUT4
Glucose transporter type 4 (GLUT4), also known as solute carrier family 2, facilitated glucose transporter member 4, is a protein encoded, in humans, by the ''SLC2A4'' gene. GLUT4 is the insulin-regulated glucose transporter found primarily in ad ...
) containing vesicles to the cell membrane, via the activation of SNARE
SNARE proteins – "Soluble NSF attachment protein, SNAP REceptors" – are a large protein family consisting of at least 24 members in yeasts and more than 60 members in mammalian and plant cells.
The primary role of SNARE proteins is to m ...
proteins, to facilitate the diffusion of glucose into the cell. PKB also phosphorylates and inhibits glycogen synthase kinase
Glycogen synthase kinase 3 (GSK-3) is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues. First discovered in 1980 as a regulatory kinase for its namesake, glycogen ...
, which is an enzyme that inhibits glycogen synthase
Glycogen synthase (UDP-glucose-glycogen glucosyltransferase) is a key enzyme in glycogenesis, the conversion of glucose into glycogen. It is a glycosyltransferase () that catalyses the reaction of UDP-glucose and (1,4--D-glucosyl)n to yield UD ...
. Therefore, PKB acts to start the process of glycogenesis, which ultimately reduces blood-glucose concentration.
Function
Regulation of gene expression
The activated IRS-1 acts as a secondary messenger within the cell to stimulate the transcription of insulin-regulated genes. First, the protein Grb2 binds the P-Tyr residue of IRS-1 in itsSH2 domain
The SH2 (Src Homology 2) domain is a structurally conserved protein domain contained within the Src oncoprotein and in many other intracellular signal-transducing proteins. SH2 domains bind to phosphorylated tyrosine residues on other proteins, ...
. Grb2
Growth factor receptor-bound protein 2, also known as Grb2, is an adaptor protein involved in signal transduction/ cell communication. In humans, the GRB2 protein is encoded by the ''GRB2'' gene.
The protein encoded by this gene binds recepto ...
is then able to bind SOS, which in turn catalyzes the replacement of bound GDP with GTP on Ras, a G protein
G proteins, also known as guanine nucleotide-binding proteins, are a Protein family, family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell (biology), ...
. This protein then begins a phosphorylation cascade, culminating in the activation of mitogen-activated protein kinase (MAPK
A mitogen-activated protein kinase (MAPK or MAP kinase) is a type of serine/threonine-specific protein kinases involved in directing cellular responses to a diverse array of stimuli, such as mitogens, osmotic stress, heat shock and proinflamm ...
), which enters the nucleus and phosphorylates various nuclear transcription factors (such as Elk1
ETS Like-1 protein Elk-1 is a protein that in humans is encoded by the ELK1. Elk-1 functions as a transcription activator. It is classified as a ternary complex factor (TCF), a subclass of the ETS family, which is characterized by a common protei ...
).
Stimulation of glycogen synthesis
Glycogen synthesis is also stimulated by the insulin receptor via IRS-1. In this case, it is theSH2 domain
The SH2 (Src Homology 2) domain is a structurally conserved protein domain contained within the Src oncoprotein and in many other intracellular signal-transducing proteins. SH2 domains bind to phosphorylated tyrosine residues on other proteins, ...
of PI-3 kinase (PI-3K) that binds the P-Tyr of IRS-1. Now activated, PI-3K can convert the membrane lipid phosphatidylinositol 4,5-bisphosphate
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)''P''2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)''P''2 is enriched at the plasma membrane where it is a substrate for a number of ...
(PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3). This indirectly activates a protein kinase, PKB ( Akt), via phosphorylation. PKB then phosphorylates several target proteins, including glycogen synthase kinase 3
Glycogen synthase kinase 3 (GSK-3) is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues. First discovered in 1980 as a regulatory kinase for its namesake, glycogen ...
(GSK-3). GSK-3 is responsible for phosphorylating (and thus deactivating) glycogen synthase. When GSK-3 is phosphorylated, it is deactivated, and prevented from deactivating glycogen synthase. In this roundabout manner, insulin increases glycogen synthesis.
Degradation of insulin
Once an insulin molecule has docked onto the receptor and effected its action, it may be released back into the extracellular environment or it may be degraded by the cell. Degradation normally involvesendocytosis
Endocytosis is a cellular process in which Chemical substance, substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a Vesicle (biology and chem ...
of the insulin-receptor complex followed by the action of insulin degrading enzyme
Insulin-degrading enzyme, also known as IDE, is an enzyme.
Known alternatively as insulysin or insulin protease, IDE is a large zinc-binding protease of the M16 metalloprotease family known to cleave multiple short polypeptides that vary conside ...
. Most insulin molecules are degraded by liver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of var ...
cells. It has been estimated that a typical insulin molecule is finally degraded about 71 minutes after its initial release into circulation.
Immune system
Besides the metabolic function, insulin receptors are also expressed on immune cells, such as macrophages, B cells, and T cells. On T cells, the expression of insulin receptors is undetectable during the resting state but up-regulated uponT-cell receptor
The T-cell receptor (TCR) is a protein complex, located on the surface of T cells (also called T lymphocytes). They are responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. ...
(TCR) activation. Indeed, insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (''INS)'' gene. It is the main Anabolism, anabolic hormone of the body. It regulates the metabol ...
has been shown when supplied exogenously to promote ''in vitro'' T cell proliferation in animal models. Insulin receptor signalling is important for maximizing the potential effect of T cells during acute infection and inflammation.
Pathology
The main activity of activation of the insulin receptor is inducing glucose uptake. For this reason "insulin insensitivity", or a decrease in insulin receptor signaling, leads todiabetes mellitus type 2
Type 2 diabetes (T2D), formerly known as adult-onset diabetes, is a form of diabetes mellitus that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent ...
– the cells are unable to take up glucose, and the result is hyperglycemia
Hyperglycemia is a condition where unusually high amount of glucose is present in blood. It is defined as blood glucose level exceeding 6.9 mmol/L (125 mg/dL) after fasting for 8 hours or 10 mmol/L (180 mg/dL) 2 hours after eating.
Blood gluc ...
(an increase in circulating glucose), and all the sequelae that result from diabetes.
Patients with insulin resistance
Insulin resistance (IR) is a pathological response in which cells in insulin-sensitive tissues in the body fail to respond normally to the hormone insulin or downregulate insulin receptors in response to hyperinsulinemia.
Insulin is a horm ...
may display acanthosis nigricans
Acanthosis nigricans is a medical sign characterised by brown-to-black, poorly defined, velvety hyperpigmentation of the skin. It is usually found in body folds, such as the posterior and lateral folds of the neck, the armpits, groin, navel, foreh ...
.
A few patients with homozygous mutations in the ''INSR'' gene have been described, which causes Donohue syndrome or Leprechaunism. This autosomal recessive
In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the Phenotype, effect of a different variant of the same gene on Homologous chromosome, the other copy of the chromosome. The firs ...
disorder results in a totally non-functional insulin receptor. These patients have low-set, often protuberant, ears, flared nostrils, thickened lips, and severe growth retardation. In most cases, the outlook for these patients is extremely poor, with death occurring within the first year of life. Other mutations of the same gene cause the less severe Rabson-Mendenhall syndrome, in which patients have characteristically abnormal teeth, hypertrophic gingiva
The gums or gingiva (: gingivae) consist of the mucosal tissue that lies over the mandible and maxilla inside the mouth. Gum health and disease can have an effect on general health.
Structure
The gums are part of the soft tissue lining of the ...
(gums), and enlargement of the pineal gland
The pineal gland (also known as the pineal body or epiphysis cerebri) is a small endocrine gland in the brain of most vertebrates. It produces melatonin, a serotonin-derived hormone, which modulates sleep, sleep patterns following the diurnal c ...
. Both diseases present with fluctuations of the glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
level: After a meal the glucose is initially very high, and then falls rapidly to abnormally low levels. Other genetic mutations to the insulin receptor gene can cause Severe Insulin Resistance.
Interactions
Insulin receptor has been shown to interact with * ENPP1, * GRB10, *GRB7
Growth factor receptor-bound protein 7, also known as GRB7, is a protein that in humans is encoded by the ''GRB7'' gene.
Function
The product of this gene belongs to a small family of Signal transducing adaptor protein, adaptor proteins that a ...
,
* IRS1
Insulin receptor substrate 1 (IRS-1) is a signaling adapter protein that in humans is encoded by the ''IRS1'' gene. It is a 180 kDa protein with amino acid sequence of 1242 residues. It contains a single pleckstrin homology (PH) domain at the N-t ...
,
* MAD2L1
Mitotic spindle assembly checkpoint protein MAD2A is a protein that in humans is encoded by the ''MAD2L1'' gene.
Function
MAD2L1 is a component of the mitotic spindle assembly checkpoint that prevents the onset of anaphase until all chromosome ...
,
* PRKCD
Protein kinase C delta type (or PKC-δ) is an enzyme that in humans is encoded by the ''PRKCD'' gene.
Function
Protein kinase C (PKC) is a family of serine- and threonine-specific protein kinases that can be activated by the second messeng ...
,
* PTPN11
Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) also known as protein-tyrosine phosphatase 1D (PTP-1D), Src homology region 2 domain-containing phosphatase-2 (SHP-2), or protein-tyrosine phosphatase 2C (PTP-2C) is an enzyme that in hu ...
, and
* SH2B1
SH2B adapter protein 1 is a protein that in humans is encoded by the ''SH2B1'' gene.
Interactions
SH2B1 has been shown to interact with:
* Grb2,
* Insulin receptor,
* Janus kinase 2, and
* TrkA.
Clinical significance
Variations close ...
.
References
Further reading
* * * * * * *External links
* * {{Portal bar, Biology, border=no Clusters of differentiation EC 2.7.10 Single-pass transmembrane proteins Tyrosine kinase receptors