Imidoyl Chloride on:
[Wikipedia]
[Google]
[Amazon]
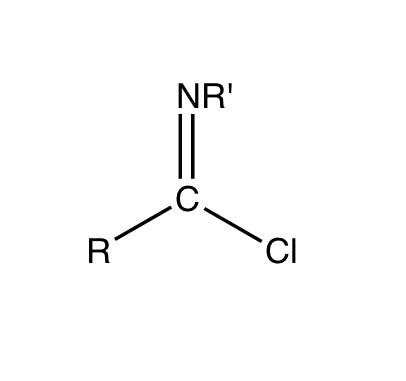 Imidoyl chlorides are organic compounds that contain the
Imidoyl chlorides are organic compounds that contain the

 Imidoyl chlorides are organic compounds that contain the
Imidoyl chlorides are organic compounds that contain the functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
RC(NR')Cl. A double bond exist between the R'N and the carbon centre. These compounds are analogues of acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example ...
. Imidoyl chlorides tend to be highly reactive and are more commonly found as intermediates in a wide variety of synthetic procedures. Such procedures include Gattermann aldehyde synthesis, Houben-Hoesch ketone synthesis, and the Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on haloimines and nitrones ...
. Their chemistry is related to that of enamines and their tautomers when the α hydrogen is next to the C=N bond.Ulrich, H. The Chemistry of Imidoyl Halides; Plenum Press: New York, 1968; pp. 55–112. Many chlorinated N-heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
s are formally imidoyl chlorides, e.g. 2-chloropyridine, 2, 4, and 6-chloropyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The othe ...
s.
Synthesis and properties
Imidoyl halides are synthesized by combining amides and halogenating agents. The structure of the carboxylic acid amides plays a role in the outcome of the synthesis. Imidoyl chloride can be prepared by treating a monosubstituted carboxylic acid amide withphosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, es ...
.
:RC(O)NHR’ + COCl2 → RC(NR’)Cl + HCl + CO2
Thionyl chloride is also used.
Imidoyl chlorides are generally colorless liquids or low-melting solids that are sensitive to both heat and especially moisture. In their IR spectra
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functiona ...
these compounds exhibit a characteristic νC=N band near 1650–1689 cm−1. Although both the syn and anti configurations are possible, most imidoyl chlorides adopt the anti configuration.

Reactivity
Imidoyl chlorides react readily with water, hydrogen sulfide, amines, and hydrogen halides. Treating imidoyl chlorides with water forms the corresponding amide: :RC(NR’)Cl + H2O → RCONHR’ + HCl Aliphatic imidoyl chlorides are more sensitive towardhydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
than aryl derivatives. Electron-withdrawing substituents decrease the reaction rate. Imidoyl chlorides react with hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The und ...
to produce thioamide
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure R–CS–NR′R″, where R, R′, and R″ are organic groups. They are analogous to amides but they exhibit greater multiple bond ch ...
s:
:RC(NR’)Cl + H2S → RC(S)NHR’ + HCl
When amines are treated with imidoyl chlorides, amidines
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines includ ...
are obtained.
:RC(NR’)Cl + 2R”NH2 → RC(NR’)NHR” + R”NH3Cl
When R' ≠ R", two isomers are possible.
Upon heating, imidoyl chlorides also undergo dehydrohalogenation to form nitriles:
:RC(NR’)Cl → RC≡N + R’Cl
Treatment of imidoyl chloride with hydrogen halides, such as HCl, forms the corresponding iminium chloride cations:
:RC(NR’)Cl + HCl → C(NHR’)Clsup>+Cl−
Applications
Imidoyl chlorides are useful intermediates in the syntheses of several compounds, including imidates, thioimidates, amidines, and imidoyl cyanides. Most of these syntheses involve replacing the chloride with alcohols, thiols, amines, and cyanates, respectively. Imidoyl chlorides can also undergo Friedel-Crafts reactions to install an imine groups on aromatic substrates. If the nitrogen of the imidoyl chloride has two substituents, the resulting chloroiminium ion is vulnerable to attack by aromatic rings without the need for a Lewis acid to remove the chloride first. This reaction is called the Vilsmeier–Haack reaction, and the chloroiminium ion is referred to as the Vilsmeier reagent. After attaching the iminium ion to the ring, the functional group can later be hydrolyzed to a carbonyl for further modification. The Vilsmeier-Haack reaction can be a useful technique to add functional groups to an aromatic ring if the ring contains electron-withdrawing groups, which make using the alternative Friedel-Crafts reaction difficult. Imidoyl chlorides can be easily halogenated at the α carbon position. By treating imidoyl chlorides with hydrogen halide, will cause all α hydrogens to be replaced with the halide. This method can be an effective way to halogenate many substances. Imidoyl chlorides can also be used to form peptide bonds by first creating amidines and then allowing them to be hydrolyzed to the amide. This approach may prove to be a useful route to synthesizing synthetic proteins. Imidoyl chlorides can be difficult to handle. Imidoyl chlorides react readily with water, which makes any attempt to isolate and store them for long periods of time difficult. Further, imidoyl chlorides tend to undergo self-condensation at higher temperatures if the imidoyl chloride has an α CH group. At even higher temperatures, the chlorine of the imidoyl chloride tends to be eliminated, leaving the nitrile. Because of these complications, imidoyl chlorides are typically prepared and used immediately. More stable intermediates are being sought, with substances such as imidoylbenzotriazoles being suggested.Katritzky, A. R.; Stevens, C. V.; Zhang, G.-F.; Jiang, J.; Kimpe, N. D. Heterocycles 1995, ''40'', 231.References
{{Reflist Functional groups Organochlorides