I-131 on:
[Wikipedia]
[Google]
[Amazon]
Iodine-131 (131I, I-131) is an important
 131I decays with a
131I decays with a ^_I -> \beta + \bar\nu_e + ^_Xe^\ast + 606 keV
:^_Xe^\ast -> ^_Xe + \gamma + 364 keV
The primary emissions of 131I decay are thus electrons with a maximal energy of 606 keV (89% abundance, others 248–807 keV) and 364 keV gamma rays (81% abundance, others 723 keV). Beta decay also produces an
 Iodine in food is absorbed by the body and preferentially concentrated in the
Iodine in food is absorbed by the body and preferentially concentrated in the
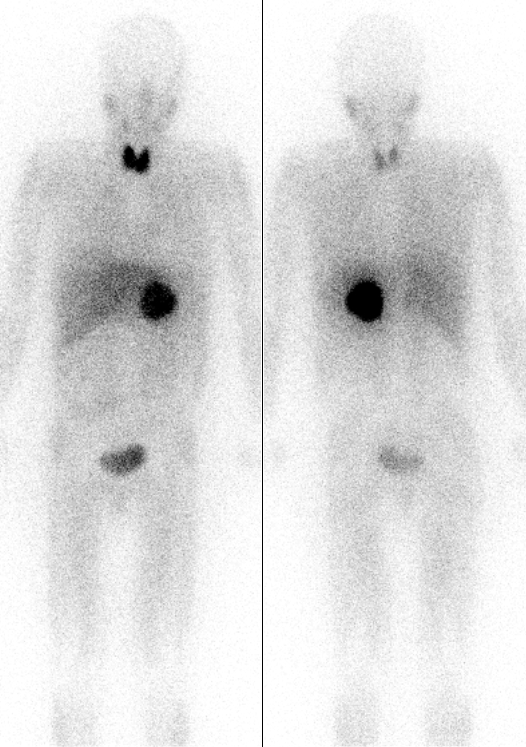 Iodine-131 is used for unsealed source radiotherapy in
Iodine-131 is used for unsealed source radiotherapy in
RadiologyInfo – The radiology information resource for patients: Radioiodine (I −131) Therapy
* [https://web.archive.org/web/20051223162858/http://rsna2004.rsna.org/rsna2004/V2004/conference/event_display.cfm?em_id=4407767 Sensitivity of Personal Homeland Security Radiation Detectors to Medical Radionuclides and Implications for Counseling of Nuclear Medicine Patients]
NLM Hazardous Substances Databank – Iodine, Radioactive
{{Thyroid hormone receptor modulators Isotopes of iodine Antithyroid drugs Fission products Radioactive contamination Medical isotopes
radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
of iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster
On 26 April 1986, the no. 4 reactor of the Chernobyl Nuclear Power Plant, located near Pripyat, Ukrainian Soviet Socialist Republic, Ukrainian SSR, Soviet Union (now Ukraine), exploded. With dozens of direct casualties, it is one of only ...
, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
of uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
and plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
, comprising nearly 3% of the total products of fission (by weight). See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233
Uranium-233 ( or U-233) is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a Nuclear fuel, reactor fuel. It has been used successfully ...
, produced from thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
.
Due to its mode of beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
, iodine-131 causes mutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, ...
and death in cells that it penetrates, and other cells up to several millimeters away. For this reason, high doses of the isotope are sometimes less dangerous than low doses, since they tend to kill thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans, it is a butterfly-shaped gland located in the neck below the Adam's apple. It consists of two connected lobes. The lower two thirds of the lobes are connected by ...
tissues that would otherwise become cancerous as a result of the radiation. For example, children treated with moderate dose of 131I for thyroid adenomas had a detectable increase in thyroid cancer
Thyroid cancer is cancer that develops from the tissues of the thyroid gland. It is a disease in which cells grow abnormally and have the potential to spread to other parts of the body. Symptoms can include swelling or a lump in the neck, ...
, but children treated with a much higher dose did not. Likewise, most studies of very-high-dose 131I for treatment of Graves' disease
Graves' disease, also known as toxic diffuse goiter or Basedow's disease, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyro ...
have failed to find any increase in thyroid cancer, even though there is linear increase in thyroid cancer risk with 131I absorption at moderate doses. Thus, iodine-131 is increasingly less employed in small doses in medical use (especially in children), but increasingly is used only in large and maximal treatment doses, as a way of killing targeted tissues. This is known as "therapeutic use".
Iodine-131 can be "seen" by nuclear medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', ...
imaging techniques (e.g., gamma camera
A gamma camera (γ-camera), also called a scintillation camera or Anger camera, is a device used to image gamma radiation emitting radioisotopes, a technique known as scintigraphy. The applications of scintigraphy include early drug development ...
s) whenever it is given for therapeutic use, since about 10% of its energy and radiation dose is via gamma radiation. However, since the other 90% of radiation (beta radiation) causes tissue damage without contributing to any ability to see or "image" the isotope, other less-damaging radioisotopes of iodine such as iodine-123
Iodine-123 (123I) is a radioactive isotope of iodine used in nuclear medicine imaging, including single-photon emission computed tomography (SPECT) or SPECT/CT exams. The isotope's half-life is 13.2232 hours; the decay by electron capture to t ...
(see isotopes of iodine
There are 40 known isotopes of iodine (53I) from 108I to 147I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.
Its longest-lived radioactive isotope, 129I, has a half-life of 16.14 million y ...
) are preferred in situations when ''only'' nuclear imaging is required. The isotope 131I is still occasionally used for purely diagnostic (i.e., imaging) work, due to its low expense compared to other iodine radioisotopes. Very small medical imaging doses of 131I have not shown any increase in thyroid cancer. The low-cost availability of 131I, in turn, is due to the relative ease of creating 131I by neutron bombardment of natural tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fou ...
in a nuclear reactor, then separating 131I out by various simple methods (i.e., heating to drive off the volatile iodine). By contrast, other iodine radioisotopes are usually created by far more expensive techniques, starting with cyclotron radiation of capsules of pressurized xenon
Xenon is a chemical element; it has symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
gas.
Iodine-131 is also one of the most commonly used gamma-emitting radioactive industrial tracer. Radioactive tracer isotopes are injected with hydraulic fracturing
Fracking (also known as hydraulic fracturing, fracing, hydrofracturing, or hydrofracking) is a well stimulation technique involving the fracturing of Formation (geology), formations in bedrock by a pressurized liquid. The process involves the ...
fluid to determine the injection profile and location of fractures created by hydraulic fracturing.Reis, John C. (1976). ''Environmental Control in Petroleum Engineering.'' Gulf Professional Publishers.
Much smaller incidental doses of iodine-131 than those used in medical therapeutic procedures, are supposed by some studies to be the major cause of increased thyroid cancers after accidental nuclear contamination. These studies suppose that cancers happen from residual tissue radiation damage caused by the 131I, and should appear mostly years after exposure, long after the 131I has decayed. Other studies did not find a correlation.
Production
Most 131I production is from neutronirradiation
Irradiation is the process by which an object is exposed to radiation. An irradiator is a device used to expose an object to radiation, most often gamma radiation, for a variety of purposes. Irradiators may be used for sterilizing medical and p ...
of a natural tellurium target in a nuclear reactor. Irradiation of natural tellurium produces almost entirely 131I as the only radionuclide with a half-life longer than hours, since most lighter isotopes of tellurium
There are 39 known isotopes and 17 nuclear isomers of tellurium (52Te), with atomic masses that range from 104 to 142. These are listed in the table below.
Naturally-occurring tellurium on Earth consists of eight isotopes. Two of these have been ...
become heavier stable isotopes, or else stable iodine or xenon. However, the heaviest naturally occurring tellurium nuclide, 130Te (34% of natural tellurium) absorbs a neutron to become tellurium-131, which beta decays with a half-life of 25 minutes to 131I.
A tellurium compound can be irradiated while bound as an oxide to an ion exchange column, with evolved 131I then eluted
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent: washing of loaded ion-exchange resins to remove captured ions, or eluting proteins or other biopolymers from an el ...
into an alkaline solution. More commonly, powdered elemental tellurium is irradiated and then 131I separated from it by dry distillation of the iodine, which has a far higher vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indicat ...
. The element is then dissolved in a mildly alkaline solution in the standard manner, to produce 131I as iodide and hypoiodate (which is soon reduced to iodide).
131I is a fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the releas ...
with a yield of 2.878% from uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
, and can be released in nuclear weapons tests and nuclear accident
A nuclear and radiation accident is defined by the International Atomic Energy Agency (IAEA) as "an event that has led to significant consequences to people, the environment or the facility." Examples include radiation poisoning, lethal effect ...
s. However, the short half-life means it is not present in significant quantities in cooled spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
, unlike iodine-129 whose half-life is nearly a billion times that of 131I.
It is discharged to the atmosphere in small quantities by some nuclear power plants.
Radioactive decay
half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 8.0249(6) days with beta minus and gamma
Gamma (; uppercase , lowercase ; ) is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter normally repr ...
emissions. This isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
of iodine has 78 neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s in its nucleus, while the only stable nuclide, 127I, has 74. On decaying, 131I most often (89% of the time) expends its 971 keV of decay energy by transforming into stable xenon-131 in two steps, with gamma decay following rapidly after beta decay:
:antineutrino
A neutrino ( ; denoted by the Greek letter ) is an elementary particle that interacts via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass is so small ('' -ino'') that it ...
, which carries off variable amounts of the beta decay energy. The electrons, due to their high mean energy (190 keV, with typical beta-decay spectra present) have a tissue penetration of .
Effects of exposure
 Iodine in food is absorbed by the body and preferentially concentrated in the
Iodine in food is absorbed by the body and preferentially concentrated in the thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans, it is a butterfly-shaped gland located in the neck below the Adam's apple. It consists of two connected lobes. The lower two thirds of the lobes are connected by ...
where it is needed for the functioning of that gland. When 131I is present in high levels in the environment from radioactive fallout, it can be absorbed through contaminated food, and will also accumulate in the thyroid. As it decays, it may cause damage to the thyroid. The primary risk from exposure to 131I is an increased risk of radiation-induced cancer
Exposure to ionizing radiation is known to increase the future incidence of cancer, particularly leukemia. The mechanism by which this occurs is well understood, but quantitative models predicting the level of risk remain controversial. The most wi ...
in later life. Other risks include the possibility of non-cancerous growths and thyroiditis.
The risk of thyroid cancer in later life appears to diminish with increasing age at time of exposure. Most risk estimates are based on studies in which radiation exposures occurred in children or teenagers. When adults are exposed, it has been difficult for epidemiologists to detect a statistically significant difference in the rates of thyroid disease above that of a similar but otherwise-unexposed group.
The risk can be mitigated by taking iodine supplements, raising the total amount of iodine in the body and, therefore, reducing uptake and retention in the face and chest and lowering the relative proportion of radioactive iodine. However, such supplements were not consistently distributed to the population living nearest to the Chernobyl nuclear power plant after the disaster, though they were widely distributed to children in Poland.
Within the US, the highest 131I fallout doses occurred during the 1950s and early 1960s to children having consumed fresh milk from sources contaminated as the result of above-ground testing of nuclear weapons. The National Cancer Institute
The National Cancer Institute (NCI) coordinates the United States National Cancer Program and is part of the National Institutes of Health (NIH), which is one of eleven agencies that are part of the U.S. Department of Health and Human Services. ...
provides additional information on the health effects from exposure to 131I in fallout, as well as individualized estimates, for those born before 1971, for each of the 3070 counties in the US. The calculations are taken from data collected regarding fallout from the nuclear weapons tests conducted at the Nevada Test Site
The Nevada National Security Sites (N2S2 or NNSS), popularized as the Nevada Test Site (NTS) until 2010, is a reservation of the United States Department of Energy located in the southeastern portion of Nye County, Nevada, about northwest of ...
.
On 27 March 2011, the Massachusetts Department of Public Health reported that 131I was detected in very low concentrations in rainwater from samples collected in Massachusetts, and that this likely originated from the Fukushima power plant. Farmers near the plant dumped raw milk, while testing in the United States found 0.8 pico- curies per liter of iodine-131 in a milk sample, but the radiation levels were 5,000 times lower than the FDA's "defined intervention level".
The levels were expected to drop relatively quickly
Treatment and prevention
A common treatment method for preventing iodine-131 exposure is by saturating the thyroid with regular, stable iodine-127, as an iodide oriodate
An iodate is the polyatomic anion with the formula . It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. Iodate salts are often colorless. They are the salts of iodic acid.
Structure
Iodate is pyra ...
salt.
Medical use
 Iodine-131 is used for unsealed source radiotherapy in
Iodine-131 is used for unsealed source radiotherapy in nuclear medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', ...
to treat several conditions. It can also be detected by gamma camera
A gamma camera (γ-camera), also called a scintillation camera or Anger camera, is a device used to image gamma radiation emitting radioisotopes, a technique known as scintigraphy. The applications of scintigraphy include early drug development ...
s for diagnostic imaging
Medical imaging is the technique and process of imaging the interior of a body for clinical analysis and medical intervention, as well as visual representation of the function of some organs or tissues (physiology). Medical imaging seeks to revea ...
, however it is rarely administered for diagnostic purposes only, imaging will normally be done following a therapeutic dose. Use of the 131I as iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
salt exploits the mechanism of absorption of iodine by the normal cells of the thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans, it is a butterfly-shaped gland located in the neck below the Adam's apple. It consists of two connected lobes. The lower two thirds of the lobes are connected by ...
gland.
Treatment of thyrotoxicosis
Major uses of 131I include the treatment of thyrotoxicosis (hyperthyroidism) due toGraves' disease
Graves' disease, also known as toxic diffuse goiter or Basedow's disease, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyro ...
, and sometimes hyperactive thyroid nodules (abnormally active thyroid tissue that is not malignant). The therapeutic use of radioiodine to treat hyperthyroidism from Graves' disease was first reported by Saul Hertz in 1941. The dose is typically administered orally (either as a liquid or capsule), in an outpatient
A patient is any recipient of health care services that are performed by healthcare professionals. The patient is most often ill or injured and in need of treatment by a physician, nurse, optometrist, dentist, veterinarian, or other healt ...
setting, and is usually 400–600 megabecquerel
The becquerel (; symbol: Bq) is the unit of radioactivity in the International System of Units (SI). One becquerel is defined as an activity of one per second, on average, for aperiodic activity events referred to a radionuclide. For applicatio ...
s (MBq). Radioactive iodine (iodine-131) alone can potentially worsen thyrotoxicosis in the first few days after treatment. One side effect of treatment is an initial period of a few days of increased hyperthyroid symptoms. This occurs because when the radioactive iodine destroys the thyroid cells, they can release thyroid hormone into the blood stream. For this reason, sometimes patients are pre-treated with thyrostatic medications such as methimazole, and/or they are given symptomatic treatment such as propranolol. Radioactive iodine treatment is contraindicated in breast-feeding and pregnancy
Treatment of thyroid cancer
Iodine-131, in higher doses than for thyrotoxicosis, is used for ablation of remnant thyroid tissue following a completethyroidectomy
A thyroidectomy is an operation that involves the surgery, surgical removal of all or part of the thyroid gland. In general surgery, endocrine or head and neck surgeons often perform a thyroidectomy when a patient has thyroid cancer or some other ...
to treat thyroid cancer
Thyroid cancer is cancer that develops from the tissues of the thyroid gland. It is a disease in which cells grow abnormally and have the potential to spread to other parts of the body. Symptoms can include swelling or a lump in the neck, ...
.
Administration of I-131 for ablation
Typical therapeutic doses of I-131 are between 2220 and 7400megabecquerel
The becquerel (; symbol: Bq) is the unit of radioactivity in the International System of Units (SI). One becquerel is defined as an activity of one per second, on average, for aperiodic activity events referred to a radionuclide. For applicatio ...
s (MBq). Because of this high radioactivity and because the exposure of stomach tissue to beta radiation would be high near an undissolved capsule, I-131 is sometimes administered to human patients in a small amount of liquid. Administration of this liquid form is usually by straw which is used to slowly and carefully suck up the liquid from a shielded container. For administration to animals (for example, cats with hyperthyroidism), for practical reasons the isotope must be administered by injection. European guidelines recommend administration of a capsule, due to "greater ease to the patient and the superior radiation protection for caregivers".
Post-treatment isolation
Ablation doses are usually administered on aninpatient
A patient is any recipient of health care services that are performed by healthcare professionals. The patient is most often ill or injured and in need of treatment by a physician, nurse, optometrist, dentist, veterinarian, or other heal ...
basis, and IAEA
The International Atomic Energy Agency (IAEA) is an intergovernmental organization that seeks to promote the peaceful use of nuclear energy and to inhibit its use for any military purpose, including nuclear weapons. It was established in 1957 ...
International Basic Safety Standards recommend that patients are not discharged until the activity falls below 1100 MBq. ICRP advice states that "comforters and carers" of patients undergoing radionuclide therapy should be treated as members of the public for dose constraint purposes and any restrictions on the patient should be designed based on this principle.
Patients receiving I-131 radioiodine treatment may be warned not to have sexual intercourse for one month (or shorter, depending on dose given), and women told not to become pregnant for six months afterwards. "This is because a theoretical risk to a developing fetus exists, even though the amount of radioactivity retained may be small and there is no medical proof of an actual risk from radioiodine treatment. Such a precaution would essentially eliminate direct fetal exposure to radioactivity and markedly reduce the possibility of conception with sperm that might theoretically have been damaged by exposure to radioiodine." These guidelines vary from hospital to hospital and will depend on national legislation and guidance, as well as the dose of radiation given. Some also advise not to hug or hold children when the radiation is still high, and a one- or two- metre distance to others may be recommended.
I-131 will be eliminated from the body over the next several weeks after it is given. The majority of I-131 will be eliminated from the human body in 3–5 days, through natural decay, and through excretion in sweat and urine. Smaller amounts will continue to be released over the next several weeks, as the body processes thyroid hormones created with the I-131. For this reason, it is advised to regularly clean toilets, sinks, bed sheets and clothing used by the person who received the treatment. Patients may also be advised to wear slippers or socks at all times, and avoid prolonged close contact with others. This minimizes accidental exposure by family members, especially children. Use of a decontaminant specially made for radioactive iodine removal may be advised. The use of chlorine bleach solutions, or cleaners that contain chlorine bleach for cleanup, are not advised, since radioactive elemental iodine gas may be released. Airborne I-131 may cause a greater risk of second-hand exposure, spreading contamination over a wide area. Patient is advised if possible to stay in a room with a bathroom connected to it to limit unintended exposure to family members.
Many airports have radiation detectors to detect the smuggling of radioactive materials. Patients should be warned that if they travel by air, they may trigger radiation detectors at airports up to 95 days after their treatment with 131I.
Other therapeutic uses
The 131I isotope is also used as a radioactive label for certainradiopharmaceutical
Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which ...
s that can be used for therapy, e.g. 131I- metaiodobenzylguanidine (131I-MIBG) for imaging and treating pheochromocytoma
Pheochromocytoma is a rare tumor of the adrenal medulla composed of chromaffin cells and is part of the paraganglioma (PGL) family of tumors, being defined as an intra-adrenal PGL. These neuroendocrine tumors can be sympathetic, where they relea ...
and neuroblastoma
Neuroblastoma (NB) is a type of cancer that forms in certain types of nerve tissue. It most frequently starts from one of the adrenal glands but can also develop in the head, neck, chest, abdomen, or Vertebral column, spine. Symptoms may include ...
. In all of these therapeutic uses, 131I destroys tissue by short-range beta radiation. About 90% of its radiation damage to tissue is via beta radiation, and the rest occurs via its gamma radiation (at a longer distance from the radioisotope). It can be seen in diagnostic scans after its use as therapy, because 131I is also a gamma-emitter.
Diagnostic uses
Because of the carcinogenicity of its beta radiation in the thyroid in small doses, I-131 is rarely used primarily or solely for diagnosis (although in the past this was more common due to this isotope's relative ease of production and low expense). Instead the more purely gamma-emitting radioiodineiodine-123
Iodine-123 (123I) is a radioactive isotope of iodine used in nuclear medicine imaging, including single-photon emission computed tomography (SPECT) or SPECT/CT exams. The isotope's half-life is 13.2232 hours; the decay by electron capture to t ...
is used in diagnostic testing (nuclear medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', ...
scan of the thyroid). The longer half-lived iodine-125
Iodine-125 (125I) is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions, including prostate cancer, uveal melanomas, and brain tumor ...
is also occasionally used when a longer half-life radioiodine is needed for diagnosis, and in brachytherapy
Brachytherapy is a form of radiation therapy where a sealed radiation, radiation source is placed inside or next to the area requiring treatment. The word "brachytherapy" comes from the Ancient Greek, Greek word , meaning "short-distance" or "s ...
treatment (isotope confined in small seed-like metal capsules), where the low-energy gamma radiation without a beta component makes iodine-125 useful. The other radioisotopes of iodine are never used in brachytherapy.
The use of 131I as a medical isotope has been blamed for a routine shipment of biosolids
Biosolids are solid organic matter recovered from a sewage treatment process and used as fertilizer. In the past, it was common for farmers to use animal manure to improve their soil fertility. In the 1920s, the farming community began also to us ...
being rejected from crossing the Canada—U.S. border. Such material can enter the sewers directly from the medical facilities, or by being excreted by patients after a treatment
Industrial radioactive tracer uses
Used for the first time in 1951 to localize leaks in a drinking water supply system ofMunich
Munich is the capital and most populous city of Bavaria, Germany. As of 30 November 2024, its population was 1,604,384, making it the third-largest city in Germany after Berlin and Hamburg. Munich is the largest city in Germany that is no ...
, Germany, iodine-131 became one of the most commonly used gamma-emitting industrial radioactive tracer
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide (a radioactive atom). By virtue of its radioactive decay, it can be used to ...
s, with applications in isotope hydrology
Isotope hydrology is a field of geochemistry and hydrology that uses naturally occurring stable and radioactive isotopic techniques to evaluate the age and origins of surface and groundwater and the processes within the atmospheric hydrologic cyc ...
and leak detection.
Since the late 1940s, radioactive tracers have been used by the oil industry. Tagged at the surface, water is then tracked downhole, using the appropriated gamma detector, to determine flows and detect underground leaks. I-131 has been the most widely used tagging isotope in an aqueous solution of sodium iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions ...
. It is used to characterize the hydraulic fracturing
Fracking (also known as hydraulic fracturing, fracing, hydrofracturing, or hydrofracking) is a well stimulation technique involving the fracturing of Formation (geology), formations in bedrock by a pressurized liquid. The process involves the ...
fluid to help determine the injection profile and location of fractures created by hydraulic fracturing
Fracking (also known as hydraulic fracturing, fracing, hydrofracturing, or hydrofracking) is a well stimulation technique involving the fracturing of Formation (geology), formations in bedrock by a pressurized liquid. The process involves the ...
.
In popular culture
*The use of iodine-131 as a poison – used in small doses over a period of time to disrupt a person's ability to think and tell right from wrong – played a central role in the episode "The Case of the Melancholy Marksman" of the long-running CBS TV series '' Perry Mason'' (season 5, episode 24, first broadcast March 24, 1962).See also
*Isotopes of iodine
There are 40 known isotopes of iodine (53I) from 108I to 147I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.
Its longest-lived radioactive isotope, 129I, has a half-life of 16.14 million y ...
* Iodine in biology
References
External links
*RadiologyInfo – The radiology information resource for patients: Radioiodine (I −131) Therapy
* [https://web.archive.org/web/20051223162858/http://rsna2004.rsna.org/rsna2004/V2004/conference/event_display.cfm?em_id=4407767 Sensitivity of Personal Homeland Security Radiation Detectors to Medical Radionuclides and Implications for Counseling of Nuclear Medicine Patients]
NLM Hazardous Substances Databank – Iodine, Radioactive
{{Thyroid hormone receptor modulators Isotopes of iodine Antithyroid drugs Fission products Radioactive contamination Medical isotopes