Hantzsch Pyrrole Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Hantzsch Pyrrole Synthesis, named for
 The mechanism starts with the amine (1) attacking the β carbon of the β-ketoesters (2), and eventually forming an
The mechanism starts with the amine (1) attacking the β carbon of the β-ketoesters (2), and eventually forming an


Arthur Rudolf Hantzsch
Arthur Rudolf Hantzsch (7 March 1857 – 14 March 1935) was a German chemist.
Life and work
Hantzsch studied chemistry in Dresden and graduated at the University of Würzburg under Johannes Wislicenus. As a professor, he taught at the Universitie ...
, is the chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
of β-ketoesters (1) with ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
(or primary amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s) and α-haloketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s (2) to give substituted pyrrole
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula . It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrol ...
s (3).
Pyrroles are found in a variety of natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
s with biological activity, so the synthesis of substituted pyrroles has important applications in medicinal chemistry. Alternative methods for synthesizing pyrroles exist, such as the Knorr Pyrrole Synthesis
The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group (e.g. an ester as shown) ...
and Paal-Knorr Synthesis.
Mechanism
Below is one published mechanism for the reaction: The mechanism starts with the amine (1) attacking the β carbon of the β-ketoesters (2), and eventually forming an
The mechanism starts with the amine (1) attacking the β carbon of the β-ketoesters (2), and eventually forming an enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the r ...
(3). The enamine then attacks the carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
carbon of the α-haloketone (4). This is followed by the loss of H2O, giving an imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
(5). This intermediate undergoes an intramolecular nucleophilic attack, forming a 5-membered ring (6). Finally, a hydrogen is eliminated and the pi-bonds are rearranged in the ring, yielding the final product (7).
An alternative mechanism has been proposed in which the enamine (3) attacks the α-carbon of the α-haloketone (4) as part of a nucleophilic substitution, instead of attacking the carbonyl carbon.Wang, Zerong. ''Comprehensive Organic Name Reactions and Reagents, 3 Volume Set''; John Wiley & Sons, Hoboken, New Jersey, 2009; pp. 1326-1327.
Generalized reaction under mechanochemical conditions
A generalization of the Hantzsch pyrrole synthesis was developed by Estevez, et al. In this reaction highly substituted pyrroles can be synthesized in a one-pot reaction, with relatively high yields (60% - 97%). This reaction involves the high-speed vibration milling (HSVM) of ketones with ''N''-iodosuccinimide (NIS) and ''p''-toluenesulfonic acid, to form an α-iodoketone ''in situ
is a Latin phrase meaning 'in place' or 'on site', derived from ' ('in') and ' ( ablative of ''situs'', ). The term typically refers to the examination or occurrence of a process within its original context, without relocation. The term is use ...
''. This is followed by addition of a primary amine, a β-dicarbonyl compound, cerium(IV) ammonium nitrate (CAN) and silver nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar causti ...
, as shown in the scheme below:

Applications
2,3-dicarbonylated pyrroles
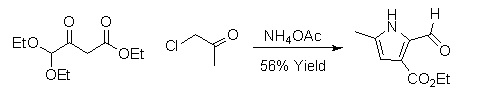
2,3-dicarbonylated pyrroles can be synthesized by a version of the Hantzsch Pyrrole Synthesis. These pyrroles are particularly useful for total synthesis because the carbonyl groups can be converted into a variety of other functional groups.
Substituted indoles
The reaction can also occur between an enamine and an α-haloketone to synthesize substitutedindole
Indole is an organic compound with the formula . Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole ...
s, which also have biological significance.
Continuous flow chemistry
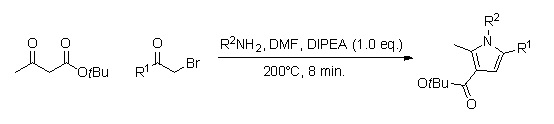
A library of substituted pyrrole analogs can be quickly produced by using continuous flow chemistry (reaction times of around 8 min.).Herath, A.; Cosford, N.D.P. '' Org. Lett.'' 2010, ''12'', 5182-5185. The advantage of using this method, as opposed to the in-flask synthesis, is that this one does not require the work-up and purification of several intermediates, and could therefore lead to a higher percent yield.
See also
*Hantzsch pyridine synthesis
The Hantzsch pyridine synthesis or Hantzsch dihydropyridine synthesis is a multi-component organic reaction between an aldehyde such as formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical for ...
References
{{Reflist Pyrroles Chemical synthesis Name reactions