Hantzsch Pyridine Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Hantzsch pyridine synthesis or Hantzsch dihydropyridine synthesis is a multi-component  The Hantzsch dihydropyridine synthesis has been affected by
The Hantzsch dihydropyridine synthesis has been affected by
 Later research using mass spectrometry monitoring with charge-tagged reactants supported intermediate pathway A as a likely route and showed evidence that the reaction followed two additional intermediate pathways which converge to precursor 7. Reagents likely influence the route taken as when the methyl group of 1 is replaced by an electron-withdrawing group, the reaction instead proceeds through a
Later research using mass spectrometry monitoring with charge-tagged reactants supported intermediate pathway A as a likely route and showed evidence that the reaction followed two additional intermediate pathways which converge to precursor 7. Reagents likely influence the route taken as when the methyl group of 1 is replaced by an electron-withdrawing group, the reaction instead proceeds through a
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
between an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
such as formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
, 2 equivalents of a β-keto ester such as ethyl acetoacetate
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds.
Preparation
At large scale, ethyl ac ...
and a nitrogen donor such as ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
or ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
. The initial reaction product is a dihydropyridine which can be oxidized
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
in a subsequent step to a pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
. The driving force for this second reaction step is aromatization
Aromatization is a chemical reaction in which an aromaticity, aromatic system is formed from a single nonaromatic precursor. Typically aromatization is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of cycl ...
. This reaction was reported in 1881 by Arthur Rudolf Hantzsch
Arthur Rudolf Hantzsch (7 March 1857 – 14 March 1935) was a German chemist.
Life and work
Hantzsch studied chemistry in Dresden and graduated at the University of Würzburg under Johannes Wislicenus. As a professor, he taught at the Universitie ...
.
A 1,4-dihydropyridine
1,4-Dihydropyridine (DHP) is an organic compound with the formula CH2(CH=CH)2NH. The parent compound is uncommon, but derivatives of 1,4-dihydropyridine are important commercially and biologically. The pervasive cofactors NADH and NADPH are deriv ...
dicarboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,... ...
is also called a 1,4-DHP compound or a Hantzsch ester. These compounds are an important class of calcium channel blocker
Calcium channel blockers (CCB), calcium channel antagonists or calcium antagonists are a group of medications that disrupt the movement of calcium () through calcium channels. Calcium channel blockers are used as antihypertensive drugs, i.e., as ...
s and as such commercialized in for instance nifedipine
Nifedipine ( ), sold under the brand name Procardia among others, is a calcium channel blocker medication used to manage angina, high blood pressure, Raynaud's phenomenon, and premature labor. It is one of the treatments of choice for Prinzme ...
, amlodipine
Amlodipine, sold under the brand name Norvasc among others, is a calcium channel blocker medication used to treat hypertension, high blood pressure, coronary artery disease (CAD) and variant angina (also called Prinzmetal angina or coronary ar ...
or nimodipine
Nimodipine, sold under the brand name Nimotop among others, is a calcium channel blocker used in preventing vasospasm secondary to subarachnoid hemorrhage (a form of cerebral hemorrhage). It was originally developed within the calcium channel b ...
.
The reaction has been demonstrated to proceed in water as reaction solvent and with direct aromatization by ferric chloride
Iron(III) chloride describes the inorganic compounds with the formula (H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated f ...
, manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cel ...
or potassium permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, which dissolves in water as K+ and ions to give an intensely pink to purple solution.
Potassium permanganate is widely us ...
in a one-pot synthesis
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction in which a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy ...
.
microwave chemistry
Microwave chemistry is the science of applying microwave radiation to chemical reactions. Microwaves act as high frequency electric fields and will generally heat any material containing mobile electric charges, such as polar molecules in a solvent ...
.
Mechanism
At least five significant pathways have been proposed for the Hantzch reaction synthesis of 1,4-dihydropyridine. Low yield and unexpected products may arise under varying reactants and reaction conditions. Previous studies have tested the reactions of preformed intermediates to determine the most likely mechanism and design successful syntheses. An early study into the mechanism using 13C and 15N NMR indicated the intermediacy of thechalcone
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids. They are widely known bioactive substances, fluorescent materi ...
6 and enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the r ...
3. This data suggested the following route for the reaction.
 Later research using mass spectrometry monitoring with charge-tagged reactants supported intermediate pathway A as a likely route and showed evidence that the reaction followed two additional intermediate pathways which converge to precursor 7. Reagents likely influence the route taken as when the methyl group of 1 is replaced by an electron-withdrawing group, the reaction instead proceeds through a
Later research using mass spectrometry monitoring with charge-tagged reactants supported intermediate pathway A as a likely route and showed evidence that the reaction followed two additional intermediate pathways which converge to precursor 7. Reagents likely influence the route taken as when the methyl group of 1 is replaced by an electron-withdrawing group, the reaction instead proceeds through a diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls ...
intermediate.
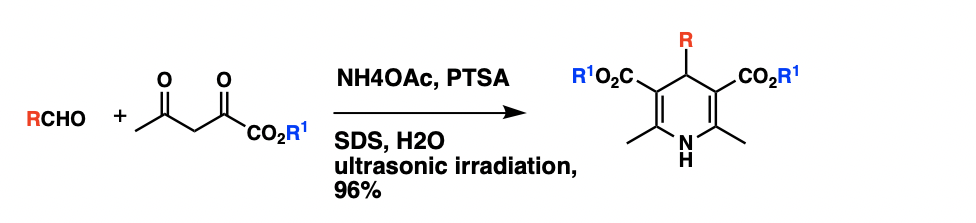
Optimization of reaction conditions
The classical method for synthesis of Hantzsch 1,4-dihydropyridines, which involves a one-pot condensation of aldehydes with ethyl acetoacetate and ammonia, have several drawbacks such as harsh reaction conditions, long reaction times, and generally low yield of products. A synthesis of 1,4-dihydropyridines in aqueous micelles catalyzed by PTSA under ultrasonic irradiation. Using condensation ofbenzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-li ...
, ethyl acetoacetate and ammonium acetate as a model, experiments have proven that when catalyzed by p-toluenesulfonic acid (PTSA) under ultrasonic irradiation, the reaction can have a product yield of 96% in aqueous (SDS, 0.1M). The reaction had also been carried out in various solvent system, and it was discovered that the ultrasonic irradiation in aqueous micelles
A micelle () or micella () ( or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated colloidal system). ...
gave better yields than in solvents such as methanol, ethanol, THF. Using the optimized reaction conditions, a series of 1,4-dihydropyridine were synthesized, and they all have a reaction yield above 90%.

Aromatization
Oxidation of 1,4-DHPs accounts for one of the easiest ways of accessing pyridine derivatives. Common oxidants used to promote aromatization of 1,4-DHPs are CrO3, KMnO4, and HNO3. However, aromatization is often accompanied by: low chemical yields, strong oxidative conditions, burdensome workups, the formation of side products, or the need of excess oxidant. As such, particular attention has been paid to developing methods of aromatization to yield pyridine derivatives under milder and efficient conditions. Such conditions include, but are not limited to:iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
in refluxing methanol, chromium dioxide
Chromium dioxide or chromium(IV) oxide is an inorganic compound with the formula CrO2. It is a black synthetic magnetic solid. It once was widely used in magnetic tape emulsion. With the increase in popularity of CDs and DVDs and more recentl ...
(CrO2), sodium chlorite
Sodium chlorite (NaClO2) is a chemical compound used in the manufacturing of paper and as a disinfectant.
Use
The main application of sodium chlorite is the generation of chlorine dioxide for bleaching and stripping of textiles, pulp, and pa ...
, and under metal-free, photochemical conditions using both UV-light and visible light. Upon metabolism, 1,4-DHP based antihypertensive drugs undergo oxidation by way of cytochrome P-450
Cytochromes P450 (P450s or CYPs) are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in ''Escherichi ...
in the liver and are thus converted to their pyridine derivatives. As a result, particular attention has been paid to the aromatization of 1,4-DHPs as a means to understand biological systems and so as to develop new methods of accessing pyridines.
Green chemistry
As amulti-component reaction A multi-component reaction (or MCR), sometimes referred to as a "Multi-component Assembly Process" (or MCAP), is a chemical reaction where three or more compounds
react to form a single product. By definition, multicomponent reactions are those re ...
, the Hantzsch pyridine synthesis is much more atom efficient with a simpler number of reaction steps than a linear-strategy synthesis. In recent years, research has looked to make this an even more environmentally friendly reaction by investigating "greener" solvents and reaction conditions. One line of study has experimented with using ionic liquids
An ionic liquid (IL) is a salt in the liquid state at ambient conditions. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are ...
as catalysts for room temperature reactions. Ionic liquids are an easy to handle and non-toxic option to replace traditional catalysts. Additionally, this catalyst lead to a high yield at room temperature, reducing the impact of heating the reaction for an extended time. A second study used ceric ammonium nitrate
Ceric ammonium nitrate (CAN) is the inorganic compound with the formula . This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard oxidant in quantitative analysis.
Preparation, properties, ...
(CAN) as an alternate catalyst and achieved a solvent-free room temperature reaction.
Knoevenagel–Fries modification
The Knoevenagel–Fries modification allows for the synthesis of unsymmetrical pyridine compounds.See also
*Hantzsch pyrrole synthesis
The Hantzsch Pyrrole Synthesis, named for Arthur Rudolf Hantzsch, is the chemical reaction of β-ketoesters (1) with ammonia (or primary amines) and α-haloketones (2) to give substituted pyrroles (3).
Pyrroles are found in a variety of natural p ...
References
{{Reflist Pyridine forming reactions Name reactions Multiple component reactions