Glycolaldehyde on:
[Wikipedia]
[Google]
[Amazon]
Glycolaldehyde is the
 In acidic or basic solution, the compound undergoes reversible tautomerization to form 1,2-dihydroxyethene.
It is the only possible diose, a 2-carbon
In acidic or basic solution, the compound undergoes reversible tautomerization to form 1,2-dihydroxyethene.
It is the only possible diose, a 2-carbon
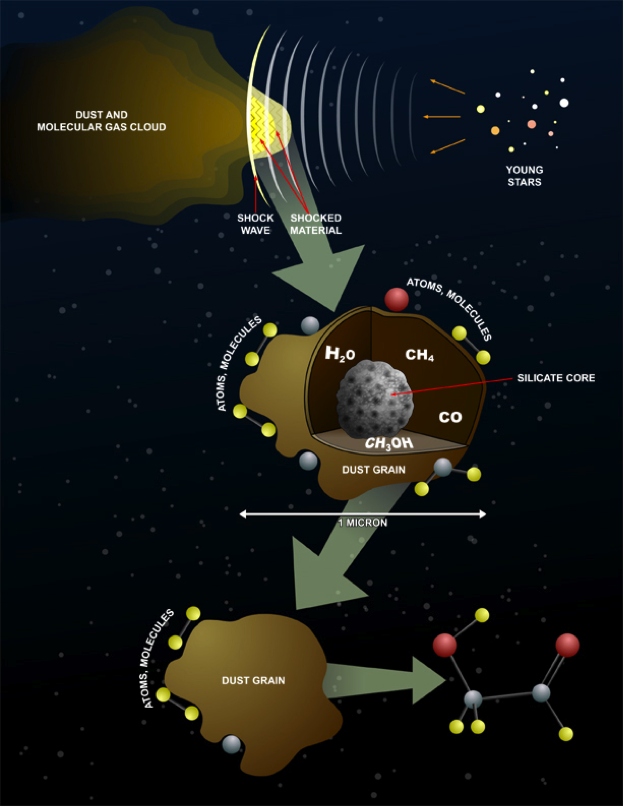 It was found that UV-irradiation of methanol ices containing CO yielded organic compounds such as glycolaldehyde and methyl formate, the more abundant isomer of glycolaldehyde. The abundances of the products slightly disagree with the observed values found in IRAS 16293-2422, but this can be accounted for by temperature changes. Ethylene Glycol and glycolaldehyde require temperatures above 30 K. The general consensus among the astrochemistry research community is in favor of the grain surface reaction hypothesis. However, some scientists believe the reaction occurs within denser and colder parts of the core. The dense core will not allow for irradiation as stated before. This change will completely alter the reaction forming glycolaldehyde.
It was found that UV-irradiation of methanol ices containing CO yielded organic compounds such as glycolaldehyde and methyl formate, the more abundant isomer of glycolaldehyde. The abundances of the products slightly disagree with the observed values found in IRAS 16293-2422, but this can be accounted for by temperature changes. Ethylene Glycol and glycolaldehyde require temperatures above 30 K. The general consensus among the astrochemistry research community is in favor of the grain surface reaction hypothesis. However, some scientists believe the reaction occurs within denser and colder parts of the core. The dense core will not allow for irradiation as stated before. This change will completely alter the reaction forming glycolaldehyde.
 The different conditions studied indicate how problematic it could be to study chemical systems that are light-years away. The conditions for the formation of glycolaldehyde are still unclear. At this time, the most consistent formation reactions seems to be on the surface of ice in
The different conditions studied indicate how problematic it could be to study chemical systems that are light-years away. The conditions for the formation of glycolaldehyde are still unclear. At this time, the most consistent formation reactions seems to be on the surface of ice in
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
with the formula . It is the smallest possible molecule that contains both an aldehyde group () and a hydroxyl group (). It is a highly reactive molecule that occurs both in the biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
and in the interstellar medium
The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It f ...
. It is normally supplied as a white solid. Although it conforms to the general formula for carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s, , it is not generally considered to be a saccharide.
Structure
Glycolaldehyde as a gas is a simple monomeric structure. As a solid and molten liquid, it exists as a dimer. Collins and George reported the equilibrium of glycolaldehyde in water by usingNMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
. In aqueous solution, it exists as a mixture of at least four species, which rapidly interconvert.
 In acidic or basic solution, the compound undergoes reversible tautomerization to form 1,2-dihydroxyethene.
It is the only possible diose, a 2-carbon
In acidic or basic solution, the compound undergoes reversible tautomerization to form 1,2-dihydroxyethene.
It is the only possible diose, a 2-carbon monosaccharide
Monosaccharides (from Greek '' monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
Chemically, monosaccharides are polyhy ...
, although a diose is not strictly a saccharide. While not a true sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecul ...
, it is the simplest sugar-related molecule. It is reported to taste sweet.
Synthesis
Glycolaldehyde is the second most abundant compound formed when preparing pyrolysis oil (up to 10% by weight). Glycolaldehyde can be synthesized by the oxidation of ethylene glycol usinghydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
in the presence of iron(II) sulfate
Iron(II) sulfate or ferrous sulfate (British English: sulphate instead of sulfate) denotes a range of salts with the formula Fe SO4·''x''H2O. These compounds exist most commonly as the heptahydrate (''x'' = 7), but several values for ...
.
Biosynthesis
It can form by action of ketolase on fructose 1,6-bisphosphate in an alternate glycolysis pathway. This compound is transferred by thiamine pyrophosphate during the pentose phosphate shunt. In purine catabolism, xanthine is first converted to urate. This is converted to 5-hydroxyisourate, which decarboxylates toallantoin
Allantoin is a chemical compound with formula C4H6N4O3. It is also called 5-ureidohydantoin or glyoxyldiureide. It is a diureide of glyoxylic acid. Allantoin is a major metabolic intermediate in most organisms including animals, plants and bacter ...
and allantoic acid. After hydrolyzing one urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
, this leaves glycolureate. After hydrolyzing the second urea, glycolaldehyde is left. Two glycolaldehydes condense to form erythrose 4-phosphate, which goes to the pentose phosphate shunt again.
Role in formose reaction
Glycolaldehyde is an intermediate in the formose reaction. In the formose reaction, two formaldehyde molecules condense to make glycolaldehyde. Glycolaldehyde then is converted to glyceraldehyde, presumably via initial tautomerization. The presence of this glycolaldehyde in this reaction demonstrates how it might play an important role in the formation of the chemical building blocks of life.Nucleotides
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
, for example, rely on the formose reaction to attain its sugar unit. Nucleotides are essential for life, because they compose the genetic information and coding for life.
Theorized role in abiogenesis
It is often invoked in theories of abiogenesis. In the laboratory, amino acids and short dipeptides have been shown to catalyze the formation of complex sugars from glycolaldehyde. For example, L-valyl-L-valine was used as a catalyst to form tetroses from glycolaldehyde. Theoretical calculations have additionally shown the feasibility of dipeptide-catalyzed synthesis of pentoses. This formation showed stereospecific, catalytic synthesis of D-ribose, the only naturally occurring enantiomer of ribose. Since the detection of this organic compound, many theories have been developed related various chemical routes to explain its formation in stellar systems. It was found that UV-irradiation of methanol ices containing CO yielded organic compounds such as glycolaldehyde and methyl formate, the more abundant isomer of glycolaldehyde. The abundances of the products slightly disagree with the observed values found in IRAS 16293-2422, but this can be accounted for by temperature changes. Ethylene Glycol and glycolaldehyde require temperatures above 30 K. The general consensus among the astrochemistry research community is in favor of the grain surface reaction hypothesis. However, some scientists believe the reaction occurs within denser and colder parts of the core. The dense core will not allow for irradiation as stated before. This change will completely alter the reaction forming glycolaldehyde.
It was found that UV-irradiation of methanol ices containing CO yielded organic compounds such as glycolaldehyde and methyl formate, the more abundant isomer of glycolaldehyde. The abundances of the products slightly disagree with the observed values found in IRAS 16293-2422, but this can be accounted for by temperature changes. Ethylene Glycol and glycolaldehyde require temperatures above 30 K. The general consensus among the astrochemistry research community is in favor of the grain surface reaction hypothesis. However, some scientists believe the reaction occurs within denser and colder parts of the core. The dense core will not allow for irradiation as stated before. This change will completely alter the reaction forming glycolaldehyde.
Formation in space
 The different conditions studied indicate how problematic it could be to study chemical systems that are light-years away. The conditions for the formation of glycolaldehyde are still unclear. At this time, the most consistent formation reactions seems to be on the surface of ice in
The different conditions studied indicate how problematic it could be to study chemical systems that are light-years away. The conditions for the formation of glycolaldehyde are still unclear. At this time, the most consistent formation reactions seems to be on the surface of ice in cosmic dust
Cosmic dustalso called extraterrestrial dust, space dust, or star dustis dust that occurs in outer space or has fallen onto Earth. Most cosmic dust particles measure between a few molecules and , such as micrometeoroids (30 μm). Cosmic dust can ...
.
Glycolaldehyde has been identified in gas and dust near the center of the Milky Way
The Milky Way or Milky Way Galaxy is the galaxy that includes the Solar System, with the name describing the #Appearance, galaxy's appearance from Earth: a hazy band of light seen in the night sky formed from stars in other arms of the galax ...
galaxy, in a star-forming region 26000 light-years from Earth, and around a protostellar binary star, IRAS 16293-2422, 400 light years from Earth. Observation of in-falling glycolaldehyde spectra 60 AU from IRAS 16293-2422 suggests that complex organic molecules may form in stellar systems prior to the formation of planets, eventually arriving on young planets early in their formation.
Detection in space
The interior region of a dust cloud is known to be relatively cold. With temperatures as cold as 4 Kelvin, the gases within the cloud will freeze and fasten themselves to the dust, which provides the reaction conditions conducive for the formation of complex molecules such as glycolaldehyde. When a star has formed from the dust cloud, the temperature within the core will increase. This will cause the molecules on the dust to evaporate and be released. The molecule will emit radio waves that can be detected and analyzed. The Atacama Large Millimeter/submillimeter Array (ALMA) first detected glycolaldehyde. ALMA consists of 66 antennas that can detect the radio waves emitted fromcosmic dust
Cosmic dustalso called extraterrestrial dust, space dust, or star dustis dust that occurs in outer space or has fallen onto Earth. Most cosmic dust particles measure between a few molecules and , such as micrometeoroids (30 μm). Cosmic dust can ...
.
On October 23, 2015, researchers at the Paris Observatory announced the discovery of glycolaldehyde and ethyl alcohol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol ...
on Comet Lovejoy, the first such identification of these substances in a comet.
References
External links
* {{Portal bar, Astronomy, Biology Primary alcohols Hydroxy aldehydes