Gattermann Aldehyde Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Gattermann reaction, (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are 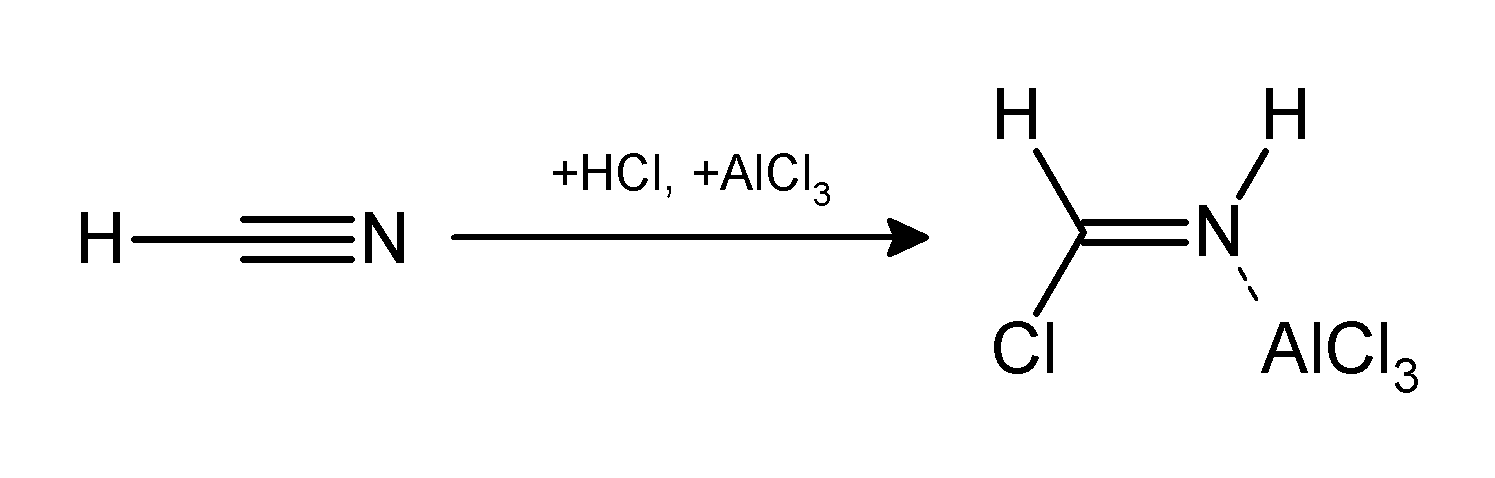
 Modifications have shown that it is possible to use
Modifications have shown that it is possible to use
 Unlike the Gattermann reaction, this reaction is not applicable to
Unlike the Gattermann reaction, this reaction is not applicable to
formylated
In biochemistry, the addition of a formyl functional group is termed formylation. A formyl functional group consists of a carbonyl bonded to hydrogen. When attached to an R group, a formyl group is called an aldehyde.
Formylation has been identi ...
by a mixture of hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on a ...
(HCN) and hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride g ...
(HCl) in the presence of a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
such as AlCl3. It is named for the German chemist Ludwig Gattermann
Ludwig Gattermann (20 April 1860 – 20 June 1920) was a German chemist who contributed significantly to both organic and inorganic chemistry.
Early life
Ludwig Gatterman was born on 20 April 1860 in Goslar, an old mining town north of the ...
and is similar to the Friedel–Crafts reaction
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactio ...
.

 Modifications have shown that it is possible to use
Modifications have shown that it is possible to use sodium cyanide
Sodium cyanide is a poisonous compound with the formula Na C N. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its h ...
or cyanogen bromide
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides (cuts the C-terminus of methionine), and synthesize other compounds. ...
in place of hydrogen cyanide.
The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide
Zinc cyanide is the inorganic compound with the formula Zinc, Zn(Cyanide, CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the organic synthesis, synthesis of organic compounds.
...
. Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN. The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(Cl)2 that serves as the Lewis-acid catalyst ''in-situ''. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenze ...
.
Gattermann–Koch reaction
The Gattermann–Koch reaction, named after the German chemists Ludwig Gattermann and Julius Arnold Koch, is a variant of the Gattermann reaction in whichcarbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
(CO) is used instead of hydrogen cyanide.
phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
and phenol ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again b ...
substrates. Although the highly unstable formyl chloride was initially postulated as an intermediate, formyl cation (i.e., protonated carbon monoxide), COsup>+, is now thought to be react directly with the arene without the initial formation of formyl chloride. Additionally, when zinc chloride is used as the Lewis acid instead of aluminum chloride for example, or when the carbon monoxide is not used at high pressure, the presence of traces of copper(I) chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear gre ...
or nickel(II) chloride
Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chem ...
co-catalyst is often necessary. The transition metal co-catalyst may server as a "carrier" by first forming reacting with CO to form a carbonyl complex, which is then transformed into the active electrophile.
See also
*Houben–Hoesch reaction
The Hoesch reaction or Houben–Hoesch reaction is an organic reaction in which a nitrile reacts with an arene compound to form an aryl ketone. The reaction is a type of Friedel-Crafts acylation with hydrogen chloride and a Lewis acid catalyst.
T ...
* Stephen aldehyde synthesis
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen (OBE/ MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and q ...
References
{{DEFAULTSORT:Gattermann-Koch reaction Substitution reactions Name reactions Formylation reactions Addition reactions Carbon-carbon bond forming reactions ru:Реакция Гаттермана - Коха