Gassman indole synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Gassman indole synthesis is a series of  This is a
This is a
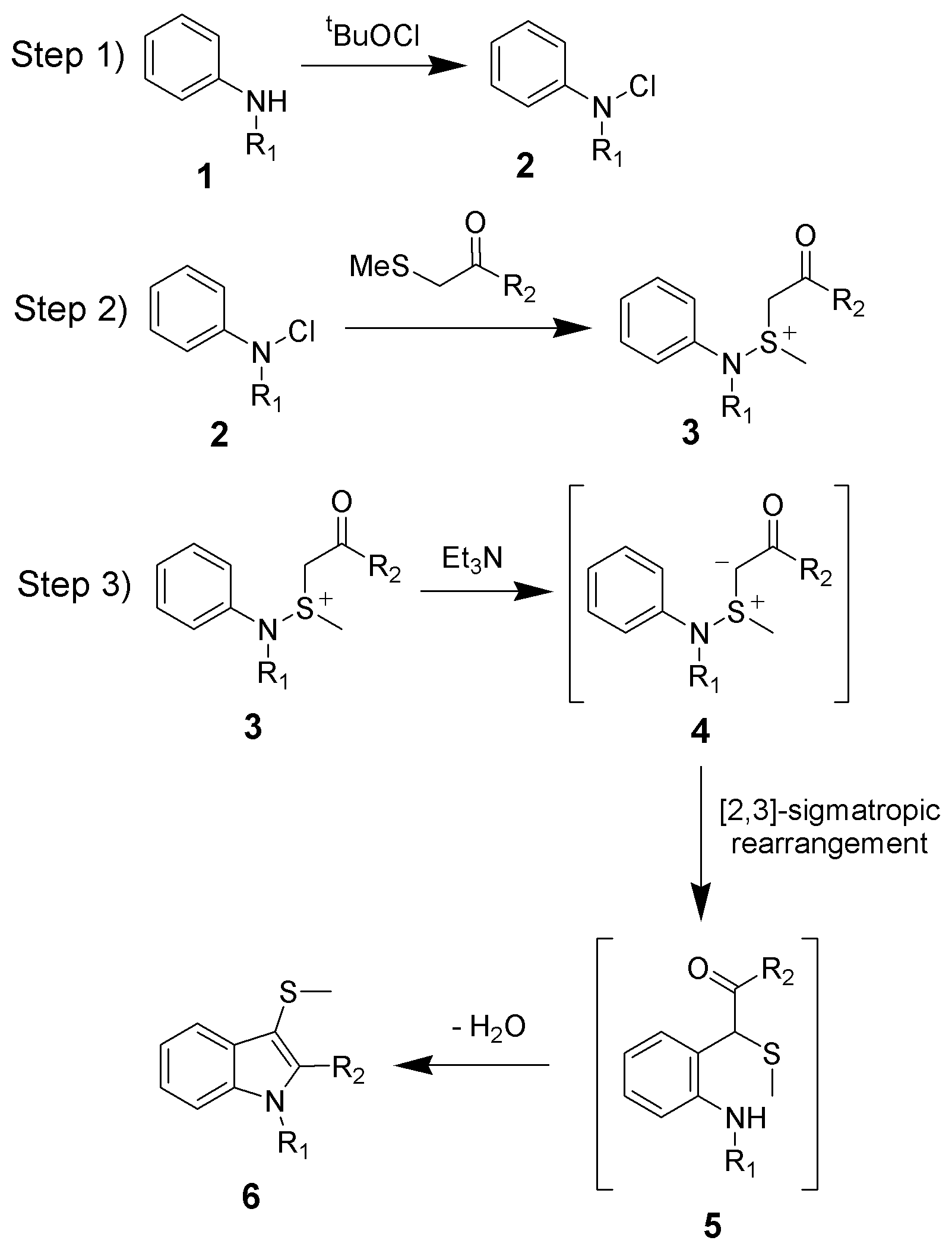 The reaction mechanism of the Gassman indole synthesis is divided among three steps.
The first step is the
The reaction mechanism of the Gassman indole synthesis is divided among three steps.
The first step is the
Article
{{DEFAULTSORT:Gassman Indole Synthesis Indole forming reactions
chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
s used to synthesize substituted indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environme ...
s by addition of an aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile start ...
and a ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
bearing a thioether substituent.
 This is a
This is a one-pot chemical reaction
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy sep ...
, and none of the intermediates are isolated. R1 can be hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
or alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloal ...
, while R2 works best with aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
, but can also be alkyl. Electron-rich anilines, such as 4-methoxyaniline, tend to fail in this reaction.
The 3-position thiomethyl group is often removed using Raney nickel to give the 3-H-indole.
Reaction mechanism
 The reaction mechanism of the Gassman indole synthesis is divided among three steps.
The first step is the
The reaction mechanism of the Gassman indole synthesis is divided among three steps.
The first step is the oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of the aniline 1 using ''tert''-butyl hypochlorite (tBuOCl) to give the chloramine
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N-H bonds have been replaced by N-Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines.
Inorganic chloramines
Inorgan ...
2.
The second step is the addition of the keto-thioether to give the sulfonium
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion (a "cation") featuring three organic substituents attached to sulfur. These organosulfur compounds have the formula . Together wit ...
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
3, and is typically done at low temperatures (−78 °C).
The third and final step is the addition of a base, which in this case is triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
. Upon warming to room temperature, the base will deprotonate
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
the sulfonium ion creating the sulfonium ylide An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both ato ...
4, which quickly undergoes a ,3sigmatropic rearrangement
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular reaction. The name ''sigmatropic'' is the result of a compounding of the long- ...
to give the ketone 5. The ketone 5 will undergo a facile condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
to give the desired 3-thiomethylindole 6.
References
* * * * *Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and exper ...
, Coll. Vol. 6, p. 601; Vol. 56, p. 72Article
{{DEFAULTSORT:Gassman Indole Synthesis Indole forming reactions