Fumarate Reductase (quinol) on:
[Wikipedia]
[Google]
[Amazon]
Fumarate reductase (quinol) (, ''QFR,'' ''FRD'', ''menaquinol-fumarate oxidoreductase, quinol:fumarate reductase'') is an 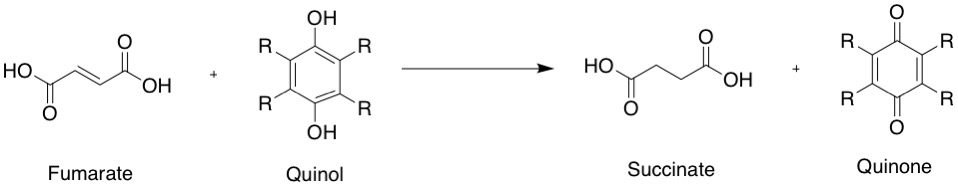 :
:


Fumarate reductase / succinate dehydrogenase FAD-binding site
in
enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
with systematic name
A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature.
A semisystematic name or semitrivi ...
''succinate:quinone oxidoreductase''. This enzyme catalyzes
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
the following chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
:
 :
: fumarate
Fumaric acid or ''trans''-butenedioic acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297.
The sa ...
+ quinol
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''para' ...
succinate
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological roles as a metabolic intermediate being converted into Fuma ...
+ quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with ...
Fumarate reductase (QFR) is a key enzyme induced by anaerobic growth of bacteria. By partaking in fumarate respiration, fumarate reductase performs the last step in the microbial anaerobic respiration. It is a membrane bound protein capable of Redox, oxidizing a quinone and passing the released electrons to an awaiting fumarate to be reduced. It is activated and synthesized under low oxygen conditions, when aerobic respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cellu ...
cannot be performed and the cell must perform anaerobic respiration
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
In aerobic organisms undergoing ...
to grow. This reaction is opposite to the reaction that is catalyzed by the related complex II of the respiratory chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this ...
(succinate dehydrogenase
Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates ...
(SQR)).
Enzyme Structure
To date, a number of QFR enzymes have been crystalized and the specifics of enzyme structure varies between organisms; however, the overall structure remains similar across different species. Fumarate reductase complexes include four subunits. Subunit A contains the site of fumarate reduction and a covalently boundflavin adenine dinucleotide
In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which ma ...
(FAD) prosthetic group. It is closely bound to subunit B, which contains three iron-sulfur centers, all placed near to each other and the nearby substrates. Subunit C consists of hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
membrane-spanning, primarily helical segments and is the site of quinol oxidization. In some fumarate reductase structures, one or more heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prostheti ...
groups are additionally bound to the C subunit and participate in the electron transfer. The D subunit contains hydrophobic alpha helices
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix).
The alpha helix is the most common structural arrangement in the secondary structure of proteins. It is also the most extreme type of l ...
that span the membrane, but does not participate in the catalytic action of the enzyme. It may be required to anchor the catalytic
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
components of the fumarate reductase complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
to the cytoplasmic membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the cytoplasm, interior of a Cell (biology), cell from the extrac ...
.

Enzyme Mechanism
The reduction of fumarate in fumarate reductase is achieved via the oxidation of a quinol bound to subunit C and the resulting transfer of electrons down a chain of iron-sulfur clusters onto a waiting FAD molecule. The edge-to-edge distances between the quinol, the iron sulfur clusters, and the FAD in this enzyme do not exceed 12.5 Angstroms and can be seen on the image below. These short distances between electron receptors allow electrons to travel down the chain at a physiologically reasonable timescale. Once electrons have travelled down the iron-sulfur clusters, they pass onto the FAD molecule bound to thecatalytic site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the ''binding si ...
of the enzyme. The final reduction of the fumarate is achieved in the active site where the asymmetrical charges from the nearby amino acids polarize the fumarate and distort its shape. Once the fumarate is no longer planar, a hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
from the bound FAD molecule in the active site attacks the double bond to reduce the fumarate. Thus, in this reaction, the fumarate serves as the terminal electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. Electron acceptors are oxidizing agents.
The electron accepting power of an electron acceptor is measured by its redox potential.
In the si ...
.

Relation to Succinate Dehydrogenase
Succinate dehydrogenase
Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates ...
(SQR) is a key enzyme in both the citric acid cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of chemical reaction, biochemical reactions that release the energy stored in nutrients through acetyl-Co ...
and the electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
in the mitochondria of eukaryotes and single celled organisms. It is a key enzyme in aerobic respiration and it performs the opposite reaction of QFR, by coupling the reduction of a quinone to the formation of succinate for use in the citric acid cycle.
Both SQR and QFR are highly related and have been shown to have some functional overlap and redundancy in various organisms. QFR and SQR are both members of the conserved protein domain family SQR_QFR_TM and have highly similar structures. It has been shown that the A and B subunits of both proteins likely evolved
Evolution is the change in the heritable Phenotypic trait, characteristics of biological populations over successive generations. It occurs when evolutionary processes such as natural selection and genetic drift act on genetic variation, re ...
from a common ancestral gene. Both enzymes have a common subunit arrangement containing a catalytic site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the ''binding si ...
, an iron-sulfur cluster containing subunit and one or two transmembrane
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently u ...
subunits with quinone binding sites and heme binding sites if applicable. Additionally, Based on a study performed in ''E. coli
''Escherichia coli'' ( )Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly foun ...
,'' researchers have concluded that under some circumstances fumarate reductase is capable of replacing succinate dehydrogenase by oxidizing succinate to produce fumarate. And it has been shown that in ''Bacillus subtilis,'' SQR is able to successfully perform the function of fumarate reductase.
Biological Function
Fumarate reductase is involved in anaerobic respiration of multiple different organisms. Most of the information gathered about fumarate reductase is from the ''Escherichia coli
''Escherichia coli'' ( )Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Escherichia'' that is commonly fo ...
'' fumarate reductase; however, fumarate reductase has also been studied in other organisms including ''Wolinella succinogenes, Helicobacter pylori
''Helicobacter pylori'', previously known as ''Campylobacter pylori'', is a gram-negative, Flagellum#bacterial, flagellated, Bacterial cellular morphologies#Helical, helical bacterium. Mutants can have a rod or curved rod shape that exhibits l ...
,'' and ''Bacteroides fragilis
''Bacteroides fragilis'' is an anaerobic, Gram-negative, pleomorphic to rod-shaped bacterium. It is part of the normal microbiota of the human colon and is generally commensal, but can cause infection if displaced into the bloodstream or surrou ...
.'' Each of these organisms has slightly different gene regulation and function in addition to different enzyme structures.
In ''E. coli,'' fumarate is the terminal electron acceptor of the energy producing electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
and fumarate reductase performs the crucial last step in this energy producing process that allows ''E. coli'' to grow when aerobic respiration and/or fermentation
Fermentation is a type of anaerobic metabolism which harnesses the redox potential of the reactants to make adenosine triphosphate (ATP) and organic end products. Organic molecules, such as glucose or other sugars, are catabolized and reduce ...
is not feasible. Because of its role in cellular energy production, its function is closely regulated by multiple conditions to ensure optimal production of energy based on current cellular needs. In addition to low oxygen conditions, fumarate reductase genes are also activated by high concentrations of fumarate and repressed in the presence of other terminal electron acceptors including nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a Cofactor (biochemistry), coenzyme central to metabolism. Found in all living cell (biology), cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphat ...
(NAD) and nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
. Nitrate suppression of fumarate reductase is common in ''E.coli'' and is carried out by two genes, narL a gene that encodes for nitrate reductase
Nitrate reductases are molybdoenzymes that reduce nitrate () to nitrite (). This reaction is critical for the production of protein in most crop plants, as nitrate is the predominant source of nitrogen in fertilized soils.
Types
Euka ...
regulator proteins and narX that encodes for a nitrate sensor protein. Other man-made antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
, including Chalcones
Chalconoids (Greek: χαλκός ''khalkós'', "copper", due to its color), also known as chalcones, are natural phenols derived from chalcone. They form the central core for a variety of important biological compounds.
They show antibacterial, ...
have also been proven to successfully inhibit fumarate reductase in addition to other cellular enzymes in order to cripple bacterial growth.
Fumarate reductase also has a notably high production of superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
and hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
in ''E. coli''. The single electron reactivity of FAD, iron-sulfur clusters, and quinones in the fumarate reductase could all contribute to electron transfer to oxygen. However, FAD has been shown to be the most significant cause of superoxide and peroxide formation in fumarate reductase, due to higher solvent accessibility in the active site than in the locations of the quinone and iron-sulfur clusters.
See also
*Succinate dehydrogenase
Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates ...
References
External links
Fumarate reductase / succinate dehydrogenase FAD-binding site
in
PROSITE
PROSITE is a protein database. It consists of entries describing the protein families, domains and functional sites as well as amino acid patterns and profiles in them. These are manually curated by a team of the Swiss Institute of Bioinformati ...
*
{{InterPro content, IPR003418
EC 1.3.5
Protein domains
Transmembrane proteins