Fullerene Ligands on:
[Wikipedia]
[Google]
[Amazon]
 A transition metal fullerene complex is a
A transition metal fullerene complex is a
file:Fullerene 4.png, h3Psub>2Pt">h3Psub>2Ptsub>6(η2-C60)
file:Fullerene 2.png, Ru3(CO)9(C60)
file:Fullerene 5.png, Platinum complex of isoxazoline-modified fullerene.
 A transition metal fullerene complex is a
A transition metal fullerene complex is a coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
wherein fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
serves as a ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
. Fullerenes
A fullerene is an allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may have hollow sphere- ...
are typically spheroidal carbon compounds, the most prevalent being buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula . It has a cage-like fused-ring structure ( truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to i ...
, C60.
One year after it was prepared in milligram quantities in 1990, C60 was shown to function as a ligand in the complex h3Psub>2Pt(η2-C60).
Since this report, a variety of transition metals
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
and binding modes were demonstrated. Most transition metal fullerene complex are derived from C60, although other fullerenes also coordinate to metals as seen with C70Rh(H)(CO)(PPh3)2.
Binding modes
As ligands, fullerenes behave similarly toelectron-deficient
In chemistry, electron deficiency (and electron-deficient) is jargon that is used in two contexts: chemical species that violate the octet rule because they have too few valence electrons and species that happen to follow the octet rule but have el ...
alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s such as tetracyanoethylene
Tetracyanoethylene (TCNE) is organic compound with the formula . It is a colorless solid, although samples are often off-white. It is an important member of the cyanocarbons.
Synthesis and reactions
TCNE is prepared by brominating malononitril ...
. Thus, their complexes are a subset of metal-alkene complex
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. The inventory is large.Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. Such compounds are intermediate ...
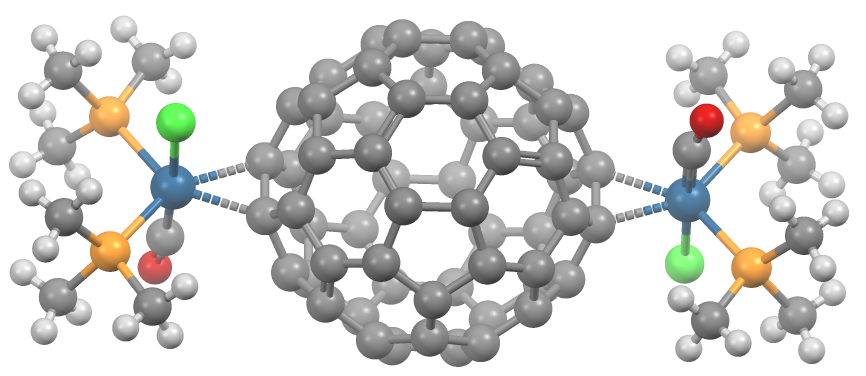
es. They almost always coordinate in a dihapto fashion and prefer electron-rich metal centers.Spessard, p. 162 This binding occurs on the junction of two 6-membered rings. Hexahapto and pentahapto bonding is rarely observed.Spessard, p. 165
In Ru3(CO)9(C60), the fullerene binds to the triangular face of the cluster.
Examples
C60 forms stable complexes of the type M(C60)(diphosphine)(CO)3 for M = Mo, W. A dirhenium complexes is known with the formula Re2(PMe3)4H8(η2:η2C60) where two of the hydrogen act as bridging ligands. Many fullerene complexes are derived from platinum metals. An unusual cationic complex features three 16e Ru centers: :3 Cp*Ru(MeCN)3+ + C60 → 3+ + 3 MeCN Vaska's complex forms a 1:1 adduct, and the analogous IrCl(CO)(PEt3)2 binds 200x more strongly. Complexes with more than one fullerene ligand are illustrated by Ir4(CO)3(μ4-CH)(PMe3)2(μ-PMe)2(CNCH2Ph)(μ-η2:η2C60)(μ4-η1:η1:η2:η2C60). In this Ir4 cluster two fullerene ligands with multiple types of mixed binding. Platinum, palladium, andnickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
form complexes of the type C60ML2 where L is a mono dentate or bidentate phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
ligand. They are prepared by displacement of weakly coordinating ligands such as ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
:
: h3Psub>2Pt(C2H4) + C60 → h3Psub>2Pt(η2-C60) + C2H4
In Et3P)2Ptsub>6(η2-C60), six Pt centers are bound to the fullerene.
Modified fullerenes as ligands
Osmium tetraoxide adds to C60 to give, in the presence of pyridine (py), the diolate C60O2OsO2(py)2. The pentaphenyl anion C60Ph5− behaves as acyclopentadienyl Cyclopentadienyl can refer to
* Cyclopentadienyl anion, or cyclopentadienide,
** Cyclopentadienyl ligand
* Cyclopentadienyl radical, •
* Cyclopentadienyl cation,
See also
* Pentadienyl
{{Chemistry index ...
ligand.
260 px, center, Ferrocene-like complex of C60Ph5−.
In this example, the binding of the ligand is similar to ferrocene
Ferrocene is an organometallic chemistry, organometallic compound with the formula . The molecule is a Cyclopentadienyl complex, complex consisting of two Cyclopentadienyl anion, cyclopentadienyl rings sandwiching a central iron atom. It is an o ...
. The anion C60(PhCH2)2Ph functions as an indenyl In organometallic chemistry, a transition metal indenyl complex is a coordination compound that contains one or more indenyl ligands. The indenyl ligand is formally the anion derived from deprotonation of indene. The η5-indenyl ligand is related ...
-like ligand.
Fullerenes can also be substituents on otherwise conventional ligands as seen with an isoxazoline fullerene chelating to platinum, rhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
, and iridium
Iridium is a chemical element; it has the symbol Ir and atomic number 77. This very hard, brittle, silvery-white transition metal of the platinum group, is considered the second-densest naturally occurring metal (after osmium) with a density ...
compounds.
Ongoing research
Although no application has been commercialized. non-linear optical (NLO) materials, and assupramolecular
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, ...
building blocks.
See also
*Exohedral fullerene
Exohedral fullerenes, also called exofullerenes, are fullerenes that have additional atoms, ions, or clusters attached their outer spheres, such as C50Cl10 and C60H8. or fullerene ligands.
See also
*Endohedral fullerene
Endohedral fullerenes, ...
*Endohedral fullerene
Endohedral fullerenes, also called endofullerenes, are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex called La@C60 was synthesized in 1985. The @ (at sign) in t ...
References
Bibliography
*Spessard, Gary; Miessler, Gary (2010). Organometallic Chemistry {{Coordination complexes Fullerenes Ligands