Fukuyama Coupling on:
[Wikipedia]
[Google]
[Amazon]
The Fukuyama coupling is a 



coupling reaction
In organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Such reactions often require the aid of a metal catalyst. In one important reaction type, a main group organometallic compound o ...
taking place between a thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix ...
and an organozinc halide in the presence of a palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
catalyst. The reaction product is a ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
. This reaction was discovered by Tohru Fukuyama
is a Japanese organic chemist and Professor of Chemistry at University of Tokyo in Japan. He discovered the Fukuyama coupling in 1998.
Biography
Fukuyama studied chemistry at Nagoya University with degrees Bachelor's (1971) and Master's (1973) ...
et al. in 1998.

Advantages
The reaction has gained considerable importance in synthetic organic chemistry due to its highchemoselectivity Chemoselectivity is the preferential reaction of a chemical reagent with one of two or more different functional groups.
In a chemoselective system, a reagent in the presence of an aldehyde and an ester would mostly target the aldehyde, even if it ...
, mild reaction conditions, and the use of less-toxic reagents. In particular, the protocol is compatible with sensitive functional groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
such as ketones, α-acetates, sulfides, aryl bromides, chlorides, and aldehydes. This excellent chemoselectivity is attributed to the fast rate of ketone formation compared to oxidative addition of palladium to aryl bromides or the nucleophilic addition of zinc reagents to aldehydes.
Mechanism
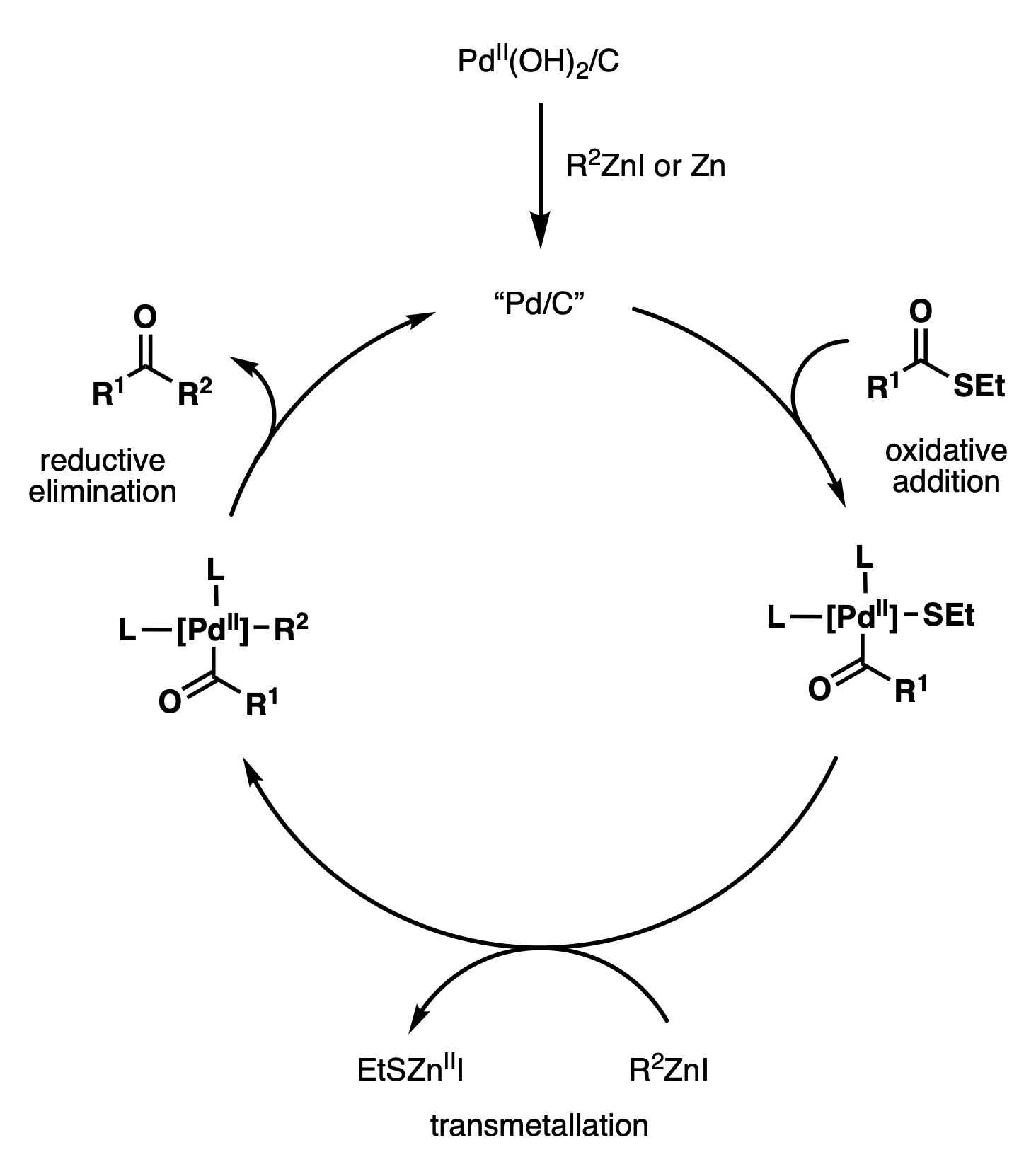
Although the Fukuyama cross-coupling reaction has been widely used in natural product synthesis, the reaction mechanism remains unclear. Various catalysts have been shown to promote reactivity, including Pd/C, Pd(OH)2/C, Pd(OAc)2, PdCl2, NiCl2, Ni(acac)2, etc. The proposed catalytic cycle using Pd(OH)2/C (Pearlman’s catalyst) features the in situ generation of active Pd/C by reduction with a zinc reagent or zinc dust. The active Pd/C species then undergoes oxidative addition with a thioester, followed bytransmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
with a zinc reagent and reductive elimination, to afford the ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
coupling product.

Reaction Conditions
Pd-catalyzed Fukuyama Coupling
Fukuyama ''et al.'' reported the PdCl2(PPh3)2-catalyzed coupling of ethyl thioesters with organozinc reagents in 1998. Remarkably, α−amino ketones starting from thioester derivatives of N-protectedamino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
can be synthesized without racemization in good to excellent yields (58-88%).

Ni-catalyzed Fukuyama Coupling
Aside from the use of palladium catalysts, the first nickel-catalyzed Fukuyama coupling was reported by Shimizu and Seki in 2002. Ni(acac)2 was found to produce superior yields compared to other nickel catalysts.
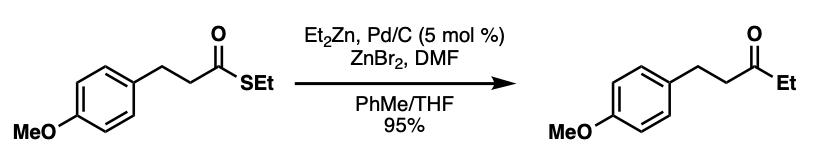
Pd/C-catalyzed Fukuyama Coupling Employing Dialkylzinc Reagents
In 2004, the same group of researchers reported the Pd/C-catalyzed Fukuyama ketone synthesis. This reaction couples dialkylzinc reagents with various thioesters in the presence of zinc bromide, which is in situ generated from bromine and zinc dust. The authors proposed that the inactive zinc bromide is shifted to the active RZnBr species via theSchlenk equilibrium The Schlenk equilibrium, named after its discoverer Wilhelm Schlenk, is a chemical equilibrium taking place in solutions of Grignard reagents and Hauser bases
:2 RMgX MgX2 + MgR2
The process described is an equilibrium between two equivalents o ...
. Additionally, DMF can be used as an additive to increase reaction yields.

Applications in Natural Product Total Synthesis
Biotin
The reaction has been used to shorten the synthesis of (+)-biotin. Previously, a lengthy sequence of six steps was required to install the C2 side chain of (+)-biotin to the thiolactone intermediate 1. Shimizu and Seki realized the efficient synthesis of (+)-biotin ''via'' the Fukuyama coupling of the thiolactone 1 and an easily prepared alkyl zinc reagent 2 in the presence of catalytic PdCl2(PPh3)2. The reaction generated an alcohol 3 which was directly reacted without purification with PTSA to afford alkene 4 in 86% yield as a single isomer.Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
and a subsequent benzyl-deprotection
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
of the alkene intermediate according to the reported procedure afforded (+)-biotin in 73% yield over two steps. This Fukuyama coupling sequence provided (+)-biotin in 63% overall yield in three steps from the thiolactone 1, thus allowing practical access to the vitamin due the short sequence, high yield, mild conditions, and ready availability of the reagents.

Related Reactions
The reaction is conceptually related toFukuyama Reduction
The Fukuyama reduction is an organic reaction and an organic reduction in which a thioester is reduced to an aldehyde by a silyl hydride in presence of a catalytic amount of palladium. This reaction was invented in 1990 by Tohru Fukuyama. In the o ...
and the Fukuyama-Mitsunobu reaction.
References
{{Reflist Carbon-carbon bond forming reactions Name reactions